当前位置:
X-MOL 学术
›
J. Label. Comp. Radiopharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Automated GMP production and long-term experience in radiosynthesis of CB1 tracer [18 F]FMPEP-d 2
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2020-07-01 , DOI: 10.1002/jlcr.3845 Salla Lahdenpohja 1 , Thomas Keller 1 , Sarita Forsback 1, 2 , Tapio Viljanen 1 , Esa Kokkomäki 1 , Riikka V Kivelä 3 , Jörgen Bergman 1 , Olof Solin 1, 2, 4 , Anna K Kirjavainen 1
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2020-07-01 , DOI: 10.1002/jlcr.3845 Salla Lahdenpohja 1 , Thomas Keller 1 , Sarita Forsback 1, 2 , Tapio Viljanen 1 , Esa Kokkomäki 1 , Riikka V Kivelä 3 , Jörgen Bergman 1 , Olof Solin 1, 2, 4 , Anna K Kirjavainen 1
Affiliation
|
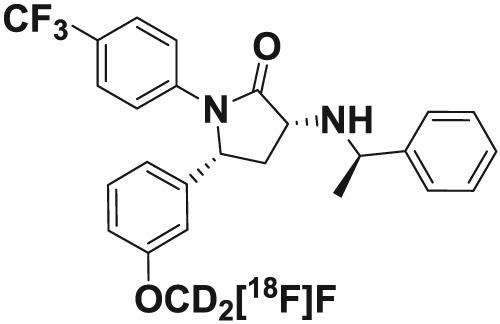
Here we describe the development of an in-house-built device for the fully automated multi-step synthesis of the cannabinoid CB1 receptor imaging tracer (3R,5R)-5-(3-([18 F]fluoromethoxy-d2 )phenyl)-3-(((R)-1-phenylethyl)amino)-1-(4-(trifluoromethyl)phenyl)pyrrolidin-2-one ([18 F]FMPEP-d2 ), following Good Manufacturing Practices. The device is interfaced to a HPLC and a sterile filtration unit in a clean room hot cell. The synthesis involves the nucleophilic 18 F-fluorination of an alkylating agent and its GC purification, the subsequent 18 F-fluoroalkylation of a precursor molecule, the semipreparative HPLC purification of the 18 F-fluoroalkylated product and its formulation for injection. We have optimized the duration and temperature of the 18 F-fluoroalkylation reaction, and addressed the radiochemical stability of the formulated product. During the past 5 years (2013 - 2018), we have performed a total of 149 syntheses for clinical use with a 90% success rate. The activity yield of the formulated product has been 1.0 ± 0.4 GBq starting from 11 ± 2 GBq and the molar activity 600 ± 300 GBq/μmol at the end of synthesis.
中文翻译:

自动化 GMP 生产和 CB1 示踪剂 [18 F]FMPEP-d 2 放射合成的长期经验
在这里,我们描述了用于全自动多步合成大麻素 CB1 受体成像示踪剂 (3R,5R)-5-(3-([18 F]氟甲氧基-d2 )苯基) 的内部构建设备的开发-3-(((R)-1-苯乙基)氨基)-1-(4-(三氟甲基)苯基)吡咯烷-2-酮([18 F]FMPEP-d2),遵循良好生产规范。该设备与洁净室热室中的 HPLC 和无菌过滤装置连接。合成过程包括烷基化剂的亲核 18 F-氟化及其 GC 纯化、随后前体分子的 18 F-氟烷基化、18 F-氟烷基化产物及其注射制剂的半制备型 HPLC 纯化。我们优化了 18 F-氟烷基化反应的持续时间和温度,并解决了配方产品的放射化学稳定性问题。在过去的5年(2013年至2018年)中,我们总共进行了149次临床合成,成功率高达90%。配制产品的活性产量从 11 ± 2 GBq 开始为 1.0 ± 0.4 GBq,合成结束时摩尔活性为 600 ± 300 GBq/μmol。
更新日期:2020-07-01
中文翻译:

自动化 GMP 生产和 CB1 示踪剂 [18 F]FMPEP-d 2 放射合成的长期经验
在这里,我们描述了用于全自动多步合成大麻素 CB1 受体成像示踪剂 (3R,5R)-5-(3-([18 F]氟甲氧基-d2 )苯基) 的内部构建设备的开发-3-(((R)-1-苯乙基)氨基)-1-(4-(三氟甲基)苯基)吡咯烷-2-酮([18 F]FMPEP-d2),遵循良好生产规范。该设备与洁净室热室中的 HPLC 和无菌过滤装置连接。合成过程包括烷基化剂的亲核 18 F-氟化及其 GC 纯化、随后前体分子的 18 F-氟烷基化、18 F-氟烷基化产物及其注射制剂的半制备型 HPLC 纯化。我们优化了 18 F-氟烷基化反应的持续时间和温度,并解决了配方产品的放射化学稳定性问题。在过去的5年(2013年至2018年)中,我们总共进行了149次临床合成,成功率高达90%。配制产品的活性产量从 11 ± 2 GBq 开始为 1.0 ± 0.4 GBq,合成结束时摩尔活性为 600 ± 300 GBq/μmol。











































 京公网安备 11010802027423号
京公网安备 11010802027423号