当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Convergent synthesis, free radical scavenging, Lineweaver‐Burk plot exploration, hemolysis and in silico study of novel indole‐phenyltriazole hybrid bearing acetamides as potent urease inhibitors
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-05-06 , DOI: 10.1002/jhet.4006 Wajiha Khan 1 , Muhammad A. Abbasi 1 , Aziz‐ur Rehman 1 , Sabahat Z. Siddiqui 1 , Majid Nazir 1 , Syed A. Ali Shah 2 , Hussain Raza 3 , Mubashir Hassan 4 , Muhammad Shahid 5 , Sung Y. Seo 3
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-05-06 , DOI: 10.1002/jhet.4006 Wajiha Khan 1 , Muhammad A. Abbasi 1 , Aziz‐ur Rehman 1 , Sabahat Z. Siddiqui 1 , Majid Nazir 1 , Syed A. Ali Shah 2 , Hussain Raza 3 , Mubashir Hassan 4 , Muhammad Shahid 5 , Sung Y. Seo 3
Affiliation
|
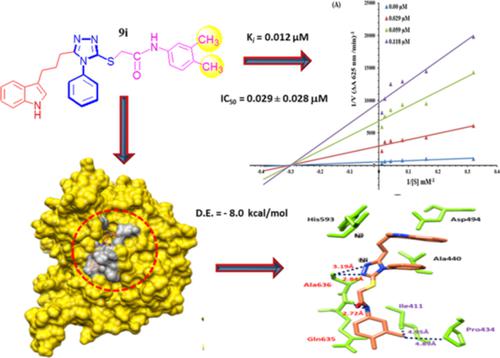
In the current paper, through a convergent multi‐step approach, a library of novel indole‐phenyltriazole hybrids containing an amide moiety (9a‐k) was synthesized. The structural verification of all synthesized molecules was accomplished by CHN and spectral analyses data. These synthesized bi‐heterocyclic derivatives (9a‐k ) were evaluated for their anti‐ulcer potential by inhibitory action against Jack bean urease enzyme and subsequently their structure‐activity relationship was perceived. Moreover, these compounds were inspected for cytotoxic profile by hemolytic activity and it was professed that nearly all the synthesized compounds showed low cytotoxicity. In addition, free radical scavenging activity and kinetic analysis were also carried out for these compounds to understand their mode of inhibition. So, it was summated that these derivatives might lead to further research gateways for obtaining better and safe anti‐ulcer agents.
中文翻译:

聚合合成,自由基清除,Lineweaver-Burk图探索,溶血和计算机模拟研究新型乙酰胺为有效尿素酶抑制剂的吲哚-苯基三唑杂化物
在当前的论文中,通过收敛的多步骤方法,合成了一个包含酰胺部分(9a-k)的新型吲哚-苯基三唑杂化物库。所有合成分子的结构验证均通过CHN和光谱分析数据完成。这些合成的双杂环衍生物(9a-k)通过对Jack bean脲酶的抑制作用评估了它们的抗溃疡潜力,随后发现了它们的结构活性关系。此外,通过溶血活性检查了这些化合物的细胞毒性特征,据信几乎所有合成的化合物都显示出低细胞毒性。此外,还对这些化合物进行了自由基清除活性和动力学分析,以了解其抑制方式。因此,可以总结为,这些衍生物可能会为获得更好和安全的抗溃疡药提供进一步的研究途径。
更新日期:2020-07-15
中文翻译:

聚合合成,自由基清除,Lineweaver-Burk图探索,溶血和计算机模拟研究新型乙酰胺为有效尿素酶抑制剂的吲哚-苯基三唑杂化物
在当前的论文中,通过收敛的多步骤方法,合成了一个包含酰胺部分(9a-k)的新型吲哚-苯基三唑杂化物库。所有合成分子的结构验证均通过CHN和光谱分析数据完成。这些合成的双杂环衍生物(9a-k)通过对Jack bean脲酶的抑制作用评估了它们的抗溃疡潜力,随后发现了它们的结构活性关系。此外,通过溶血活性检查了这些化合物的细胞毒性特征,据信几乎所有合成的化合物都显示出低细胞毒性。此外,还对这些化合物进行了自由基清除活性和动力学分析,以了解其抑制方式。因此,可以总结为,这些衍生物可能会为获得更好和安全的抗溃疡药提供进一步的研究途径。










































 京公网安备 11010802027423号
京公网安备 11010802027423号