当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protonation of CH3 N3 and CF3 N3 in Superacids: Isolation and Structural Characterization of Long-Lived Methyl- and Trifluoromethylamino Diazonium Ions.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-06 , DOI: 10.1002/anie.202002750 Thomas Saal 1 , Zsófia E Blastik 2, 3 , Ralf Haiges 1 , Archith Nirmalchandar 1 , Amanda F Baxter 1 , Karl O Christe 1 , Monica Vasiliu 4 , David A Dixon 4 , Petr Beier 2 , G K Surya Prakash 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-06 , DOI: 10.1002/anie.202002750 Thomas Saal 1 , Zsófia E Blastik 2, 3 , Ralf Haiges 1 , Archith Nirmalchandar 1 , Amanda F Baxter 1 , Karl O Christe 1 , Monica Vasiliu 4 , David A Dixon 4 , Petr Beier 2 , G K Surya Prakash 1
Affiliation
|
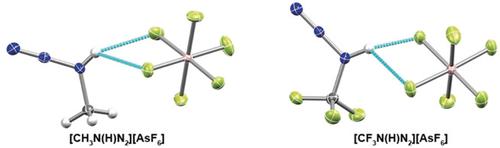
The methylamino diazonium cations [CH3N(H)N2]+ and [CF3N(H)N2]+ were prepared as their low‐temperature stable [AsF6]− salts by protonation of azidomethane and azidotrifluoromethane in superacidic systems. They were characterized by NMR and Raman spectroscopy. Unequivocal proof of the protonation site was obtained by the crystal structures of both salts, confirming the formation of alkylamino diazonium ions. The Lewis adducts CH3N3⋅AsF5 and CF3N3⋅AsF5 were also prepared and characterized by low‐temperature NMR and Raman spectroscopy, and also by X‐ray structure determination for CH3N3⋅AsF5. Electronic structure calculations were performed to provide additional insights. Attempted electrophilic amination of aromatics such as benzene and toluene with methyl‐ and trifluoromethylamino diazonium ions were unsuccessful.
中文翻译:

CH3 N3和CF3 N3在超强酸中的质子化:长寿命的甲基和三氟甲基氨基重氮鎓离子的分离和结构表征。
通过在超酸性体系中叠氮甲烷和叠氮三氟甲烷的质子化反应,制备了低温稳定的[AsF 6 ] -盐,制备了甲基氨基重氮阳离子[CH 3 N(H)N 2 ] +和[CF 3 N(H)N 2 ] +。。它们通过NMR和拉曼光谱法表征。通过两种盐的晶体结构获得质子化位点的明确证据,证实了烷基氨基重氮离子的形成。刘易斯加合物CH 3 N 3· AsF 5和CF 3 N 3· AsF 5还制备和表征通过低温NMR和拉曼光谱,并且还通过X射线结构确定为CH 3 Ñ 3 ⋅AsF 5。进行电子结构计算以提供更多的见解。尝试用甲基和三氟甲基氨基重氮离子对苯和甲苯等芳香族化合物进行亲电胺化反应失败。
更新日期:2020-07-13
中文翻译:

CH3 N3和CF3 N3在超强酸中的质子化:长寿命的甲基和三氟甲基氨基重氮鎓离子的分离和结构表征。
通过在超酸性体系中叠氮甲烷和叠氮三氟甲烷的质子化反应,制备了低温稳定的[AsF 6 ] -盐,制备了甲基氨基重氮阳离子[CH 3 N(H)N 2 ] +和[CF 3 N(H)N 2 ] +。。它们通过NMR和拉曼光谱法表征。通过两种盐的晶体结构获得质子化位点的明确证据,证实了烷基氨基重氮离子的形成。刘易斯加合物CH 3 N 3· AsF 5和CF 3 N 3· AsF 5还制备和表征通过低温NMR和拉曼光谱,并且还通过X射线结构确定为CH 3 Ñ 3 ⋅AsF 5。进行电子结构计算以提供更多的见解。尝试用甲基和三氟甲基氨基重氮离子对苯和甲苯等芳香族化合物进行亲电胺化反应失败。











































 京公网安备 11010802027423号
京公网安备 11010802027423号