当前位置:
X-MOL 学术
›
J. Trace Elem. Med. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Possible differences in the mechanism of malignant transformation of HaCaT cells by arsenite and its dimethyl metabolites, particularly dimethylthioarsenics.
Journal of Trace Elements in Medicine and Biology ( IF 3.6 ) Pub Date : 2020-05-06 , DOI: 10.1016/j.jtemb.2020.126544 Jiayuan Mao 1 , Qianlei Yang 1 , Makoto Miyazawa 2 , Motofumi Miura 2 , Luna Wang 1 , Haixuan Xia 1 , Koichi Kato 2 , Kenzo Yamanaka 2 , Yan An 1
Journal of Trace Elements in Medicine and Biology ( IF 3.6 ) Pub Date : 2020-05-06 , DOI: 10.1016/j.jtemb.2020.126544 Jiayuan Mao 1 , Qianlei Yang 1 , Makoto Miyazawa 2 , Motofumi Miura 2 , Luna Wang 1 , Haixuan Xia 1 , Koichi Kato 2 , Kenzo Yamanaka 2 , Yan An 1
Affiliation
|
BACKGROUND
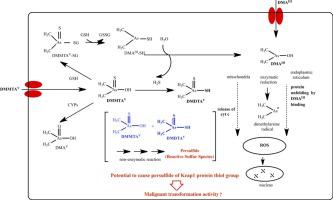
As a confirmed human carcinogen, arsenic can cause skin cancer, lung cancer, etc. However, its carcinogenic mechanism is still unclear. In recent years, the oxidative stress hypothesis has become widely accepted. In mammals it has been found that arsenic can be converted to dimethylarsinous acid (DMAIII) and dimethylmonothioarsinic acid (DMMTAV) through a series of methylation and redox reactions. DMAIII and DMMTAV are highly toxic.
METHODS
Human keratinocytes (HaCaT) were exposed to different concentrations of NaAsO2 (IAsIII), DMMTAV and DMAIII for 24 h. Reactive oxygen species (hydrogen peroxide and superoxide), oxidative damage markers (8-hydroxydeoxyguanosine and malondialdehyde), and antioxidant markers (glutathione and superoxide dismutase) were measured. In addition, sulfane sulfurs were measured in HaCaT cells and a cell-free system.
RESULTS
In the DMMTAV and DMAIII treatment groups, the levels of hydrogen peroxide and superoxide in HaCaT cells were higher than in the IAsIII treatment groups at the same dose. Levels of 8-OHdG and MDA in the DMMTAV and DMAIII treatment groups were also higher than those in the IAsIII treatment groups at the same dose. However, in the DMMTAV and DMAIII treatment groups, the levels of GSH and SOD activity were lower than that in the IAsIII treatment groups. In DMMTAV-treated HaCaT cells, sulfane sulfurs were produced. Further, it was found that DMMTAV could react with DMDTAV to form persulfide in the cell-free system, which may explain the mechanism of the formation of sulfane sulfurs in DMMTAV-treated HaCaT cells.
CONCLUSIONS
DMMTAV and DMAIII more readily induce reactive oxygen species (ROS) and cause oxidative damage in HaCaT cells than inorganic arsenic. Further, the persulfide formed by the reaction of DMMTAV and DMDTAV produced from the metabolism of DMMTAV may induce a stronger reductive defense mechanism than GSH against the intracellular oxidative stress of DMMTAV. However, the cells exposed to arsenite are transformed by the continuous nuclear translocation of Nrf2 due to oxidative stress, and the persulfide from dimethylthioarsenics may promote Nrf2 by the combination with thiol groups, especially redox control key protein, Keap1, eventually cause nuclear translocation of sustained Nrf2.
中文翻译:

亚砷酸盐及其二甲基代谢物,特别是二甲硫代砷对 HaCaT 细胞的恶性转化机制可能存在差异。
背景技术砷作为已被证实的人类致癌物,可引起皮肤癌、肺癌等,但其致癌机制尚不清楚。近年来,氧化应激假说已被广泛接受。在哺乳动物中,人们发现砷可以通过一系列甲基化和氧化还原反应转化为二甲基胂酸(DMAIII)和二甲基一硫代胂酸(DMMTAV)。 DMAIII 和 DMMTAV 具有剧毒。方法将人角质形成细胞(HaCaT)暴露于不同浓度的NaAsO2(IAsIII)、DMMTAV和DMAIII中24小时。测量了活性氧(过氧化氢和超氧化物)、氧化损伤标记物(8-羟基脱氧鸟苷和丙二醛)和抗氧化标记物(谷胱甘肽和超氧化物歧化酶)。此外,在 HaCaT 细胞和无细胞系统中测量了硫烷硫。结果 DMMTAV和DMAIII治疗组中,HaCaT细胞中过氧化氢和超氧化物水平高于相同剂量的IAsIII治疗组。 DMMTAV和DMAIII治疗组的8-OHdG和MDA水平也高于相同剂量的IAsIII治疗组。然而,在DMMTAV和DMAIII治疗组中,GSH和SOD活性水平低于IAsIII治疗组。在 DMMTAV 处理的 HaCaT 细胞中,产生硫烷硫。此外,还发现DMMTAV可以在无细胞系统中与DMDTAV反应形成过硫化物,这可能解释了DMMTAV处理的HaCaT细胞中硫烷硫的形成机制。结论 DMMTAV 和 DMAIII 比无机砷更容易诱导活性氧 (ROS) 并导致 HaCaT 细胞氧化损伤。 此外,DMMTAV与DMMTAV代谢产生的DMDTAV反应形成的过硫化物可能诱导比GSH更强的针对DMMTAV细胞内氧化应激的还原防御机制。然而,暴露于亚砷酸盐的细胞由于氧化应激而被Nrf2持续核转位而转化,二甲基硫代砷中的过硫化物可能通过与硫醇基团特别是氧化还原控制关键蛋白Keap1的结合促进Nrf2,最终导致持续的核转位。 Nrf2。
更新日期:2020-05-06
中文翻译:

亚砷酸盐及其二甲基代谢物,特别是二甲硫代砷对 HaCaT 细胞的恶性转化机制可能存在差异。
背景技术砷作为已被证实的人类致癌物,可引起皮肤癌、肺癌等,但其致癌机制尚不清楚。近年来,氧化应激假说已被广泛接受。在哺乳动物中,人们发现砷可以通过一系列甲基化和氧化还原反应转化为二甲基胂酸(DMAIII)和二甲基一硫代胂酸(DMMTAV)。 DMAIII 和 DMMTAV 具有剧毒。方法将人角质形成细胞(HaCaT)暴露于不同浓度的NaAsO2(IAsIII)、DMMTAV和DMAIII中24小时。测量了活性氧(过氧化氢和超氧化物)、氧化损伤标记物(8-羟基脱氧鸟苷和丙二醛)和抗氧化标记物(谷胱甘肽和超氧化物歧化酶)。此外,在 HaCaT 细胞和无细胞系统中测量了硫烷硫。结果 DMMTAV和DMAIII治疗组中,HaCaT细胞中过氧化氢和超氧化物水平高于相同剂量的IAsIII治疗组。 DMMTAV和DMAIII治疗组的8-OHdG和MDA水平也高于相同剂量的IAsIII治疗组。然而,在DMMTAV和DMAIII治疗组中,GSH和SOD活性水平低于IAsIII治疗组。在 DMMTAV 处理的 HaCaT 细胞中,产生硫烷硫。此外,还发现DMMTAV可以在无细胞系统中与DMDTAV反应形成过硫化物,这可能解释了DMMTAV处理的HaCaT细胞中硫烷硫的形成机制。结论 DMMTAV 和 DMAIII 比无机砷更容易诱导活性氧 (ROS) 并导致 HaCaT 细胞氧化损伤。 此外,DMMTAV与DMMTAV代谢产生的DMDTAV反应形成的过硫化物可能诱导比GSH更强的针对DMMTAV细胞内氧化应激的还原防御机制。然而,暴露于亚砷酸盐的细胞由于氧化应激而被Nrf2持续核转位而转化,二甲基硫代砷中的过硫化物可能通过与硫醇基团特别是氧化还原控制关键蛋白Keap1的结合促进Nrf2,最终导致持续的核转位。 Nrf2。











































 京公网安备 11010802027423号
京公网安备 11010802027423号