当前位置:
X-MOL 学术
›
J. Anal. Appl. Pyrol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation Mechanism of Zinc Ferrite in High-Temperature Roasting of Zinc Oxide and Ferric Oxide
Journal of Analytical and Applied Pyrolysis ( IF 5.8 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.jaap.2020.104832 Yunyan Wang , Limin Zhang , Ning Peng , Xu Yan , Yanjie Liang , Qinglin Pan , Guomin Jiang
Journal of Analytical and Applied Pyrolysis ( IF 5.8 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.jaap.2020.104832 Yunyan Wang , Limin Zhang , Ning Peng , Xu Yan , Yanjie Liang , Qinglin Pan , Guomin Jiang
|
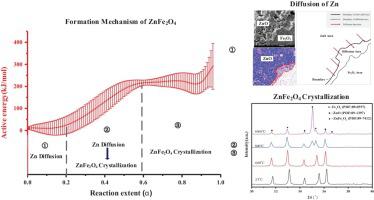
Abstract The formation mechanism of zinc ferrite during the heat treatment of zinc oxide and ferric oxide was investigated. It is revealed in the kinetic study that the formation of zinc ferrite is an endothermic reaction with an apparent activation energy Ea that increases from 9.76 kJ mol−1 to 290.70 kJ mol−1. This variation of Ea indicates that the reaction between ZnO and Fe2O3 is a multistep reaction, which could be described by the D3 (3D Jander Equation) - An (n-dimension Avrami-Erofeev Equation) model. The first step is the diffusion of the reactant, corresponding to the stage when the Ea is low. As the reaction proceeds, the rate-determining process switches to the crystallization of zinc ferrite, which is reflected by a simultaneous increase in the XRD peak height and Ea. The results of first-princeples calculations show that zinc oxide preferentially diffuses to the iron oxide matrix, and the inverse process causes an increase in the system energy. These results provide great insight into the kinetic basis for understanding the formation of spinel ferrites via a high-temperature solid-state reaction.
中文翻译:

氧化锌和三氧化二铁高温焙烧中铁酸锌的形成机理
摘要 研究了氧化锌和氧化铁热处理过程中铁酸锌的形成机理。动力学研究表明,铁酸锌的形成是吸热反应,表观活化能 Ea 从 9.76 kJ mol-1 增加到 290.70 kJ mol-1。Ea 的这种变化表明 ZnO 和 Fe2O3 之间的反应是一个多步反应,可以用 D3(3D Jander 方程)-An(n 维 Avrami-Erofeev 方程)模型来描述。第一步是反应物的扩散,对应Ea低的阶段。随着反应的进行,速率决定过程切换到铁酸锌的结晶,这反映在 XRD 峰高和 Ea 的同时增加。第一性原理计算结果表明,氧化锌优先扩散到氧化铁基体中,逆过程导致系统能量增加。这些结果为理解通过高温固态反应形成尖晶石铁氧体的动力学基础提供了很好的见解。
更新日期:2020-08-01
中文翻译:

氧化锌和三氧化二铁高温焙烧中铁酸锌的形成机理
摘要 研究了氧化锌和氧化铁热处理过程中铁酸锌的形成机理。动力学研究表明,铁酸锌的形成是吸热反应,表观活化能 Ea 从 9.76 kJ mol-1 增加到 290.70 kJ mol-1。Ea 的这种变化表明 ZnO 和 Fe2O3 之间的反应是一个多步反应,可以用 D3(3D Jander 方程)-An(n 维 Avrami-Erofeev 方程)模型来描述。第一步是反应物的扩散,对应Ea低的阶段。随着反应的进行,速率决定过程切换到铁酸锌的结晶,这反映在 XRD 峰高和 Ea 的同时增加。第一性原理计算结果表明,氧化锌优先扩散到氧化铁基体中,逆过程导致系统能量增加。这些结果为理解通过高温固态反应形成尖晶石铁氧体的动力学基础提供了很好的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号