当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Genes and lipids that impact uptake and assimilation of exogenous coenzyme Q in Saccharomyces cerevisiae.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-05-06 , DOI: 10.1016/j.freeradbiomed.2020.04.029 Lucía Fernández-Del-Río 1 , Miranda E Kelly 1 , Jaime Contreras 1 , Michelle C Bradley 1 , Andrew M James 2 , Michael P Murphy 3 , Gregory S Payne 4 , Catherine F Clarke 1
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-05-06 , DOI: 10.1016/j.freeradbiomed.2020.04.029 Lucía Fernández-Del-Río 1 , Miranda E Kelly 1 , Jaime Contreras 1 , Michelle C Bradley 1 , Andrew M James 2 , Michael P Murphy 3 , Gregory S Payne 4 , Catherine F Clarke 1
Affiliation
|
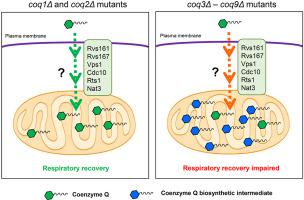
Coenzyme Q (CoQ) is an essential player in the respiratory electron transport chain and is the only lipid-soluble antioxidant synthesized endogenously in mammalian and yeast cells. In humans, genetic mutations, pathologies, certain medical treatments, and aging, result in CoQ deficiencies, which are linked to mitochondrial, cardiovascular, and neurodegenerative diseases. The only strategy available for these patients is CoQ supplementation. CoQ supplements benefit a small subset of patients, but the poor solubility of CoQ greatly limits treatment efficacy. Consequently, the efficient delivery of CoQ to the mitochondria and restoration of respiratory function remains a major challenge. A better understanding of CoQ uptake and mitochondrial delivery is crucial to make this molecule a more efficient and effective therapeutic tool. In this study, we investigated the mechanism of CoQ uptake and distribution using the yeast Saccharomyces cerevisiae as a model organism. The addition of exogenous CoQ was tested for the ability to restore growth on non-fermentable medium in several strains that lack CoQ synthesis (coq mutants). Surprisingly, we discovered that the presence of CoQ biosynthetic intermediates impairs assimilation of CoQ into a functional respiratory chain in yeast cells. Moreover, a screen of 40 gene deletions considered to be candidates to prevent exogenous CoQ from rescuing growth of the CoQ-less coq2Δ mutant, identified six novel genes (CDC10, RTS1, RVS161, RVS167, VPS1, and NAT3) as necessary for efficient trafficking of CoQ to mitochondria. The proteins encoded by these genes represent essential steps in the pathways responsible for transport of exogenously supplied CoQ to its functional sites in the cell, and definitively associate CoQ distribution with endocytosis and intracellular vesicular trafficking pathways conserved from yeast to human cells.
中文翻译:

影响酿酒酵母中外源辅酶 Q 摄取和同化的基因和脂质。
辅酶 Q (CoQ) 是呼吸电子传递链中的重要参与者,并且是哺乳动物和酵母细胞内源性合成的唯一脂溶性抗氧化剂。在人类中,基因突变、病理、某些医学治疗和衰老会导致 CoQ 缺乏,这与线粒体、心血管和神经退行性疾病有关。这些患者唯一可用的策略是补充辅酶Q。辅酶Q补充剂使一小部分患者受益,但辅酶Q的溶解度差极大地限制了治疗效果。因此,CoQ 向线粒体的有效传递和呼吸功能的恢复仍然是一项重大挑战。更好地了解 CoQ 摄取和线粒体传递对于使这种分子成为更有效和更有效的治疗工具至关重要。在这项研究中,我们使用酵母酿酒酵母作为模式生物研究了 CoQ 摄取和分布的机制。测试了添加外源 CoQ 在一些缺乏 CoQ 合成的菌株(coq 突变体)中恢复在不可发酵培养基上生长的能力。令人惊讶的是,我们发现 CoQ 生物合成中间体的存在会损害 CoQ 同化到酵母细胞中的功能性呼吸链中。此外,筛选 40 个基因缺失被认为是阻止外源 CoQ 拯救无 CoQ 的 coq2Δ 突变体生长的候选基因,确定了六个新基因(CDC10、RTS1、RVS161、RVS167、VPS1 和 NAT3)是有效贩运所必需的CoQ 对线粒体的影响。
更新日期:2020-05-06
中文翻译:

影响酿酒酵母中外源辅酶 Q 摄取和同化的基因和脂质。
辅酶 Q (CoQ) 是呼吸电子传递链中的重要参与者,并且是哺乳动物和酵母细胞内源性合成的唯一脂溶性抗氧化剂。在人类中,基因突变、病理、某些医学治疗和衰老会导致 CoQ 缺乏,这与线粒体、心血管和神经退行性疾病有关。这些患者唯一可用的策略是补充辅酶Q。辅酶Q补充剂使一小部分患者受益,但辅酶Q的溶解度差极大地限制了治疗效果。因此,CoQ 向线粒体的有效传递和呼吸功能的恢复仍然是一项重大挑战。更好地了解 CoQ 摄取和线粒体传递对于使这种分子成为更有效和更有效的治疗工具至关重要。在这项研究中,我们使用酵母酿酒酵母作为模式生物研究了 CoQ 摄取和分布的机制。测试了添加外源 CoQ 在一些缺乏 CoQ 合成的菌株(coq 突变体)中恢复在不可发酵培养基上生长的能力。令人惊讶的是,我们发现 CoQ 生物合成中间体的存在会损害 CoQ 同化到酵母细胞中的功能性呼吸链中。此外,筛选 40 个基因缺失被认为是阻止外源 CoQ 拯救无 CoQ 的 coq2Δ 突变体生长的候选基因,确定了六个新基因(CDC10、RTS1、RVS161、RVS167、VPS1 和 NAT3)是有效贩运所必需的CoQ 对线粒体的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号