Tetrahedron ( IF 2.1 ) Pub Date : 2020-05-06 , DOI: 10.1016/j.tet.2020.131253 Stephen W. Wright , Brittany Simpson , Gary Chinigo , Matthew A. Perry , Robert J. Maguire
|
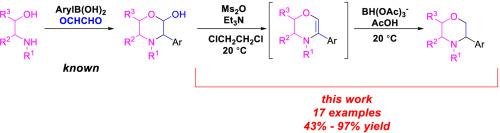
2-Hydroxy-3-aryl morpholines are readily prepared from arylboronic acids, aqueous glyoxal, and 1,2-aminoethanols by a variant of the Petasis borono-Mannich reaction. We now show that the 2-hydroxy-3-aryl morpholines may be deoxygenated to 3-aryl morpholines by treatment with methanesulfonic anhydride and triethylamine to afford intermediate 3,4-dihydro-2H-1,4-oxazines, followed by reaction with a triacetoxyborohydride salt and acetic acid to afford 3-aryl morpholines. This reaction sequence constitutes a three step, “two pot” preparation of 3-aryl morpholines from readily available starting materials (1,2-aminoethanols, arylboronic acids, and glyoxal) with excellent functional group tolerance and adaptability to scale.
中文翻译:

将2-羟基-3-芳基吗啉还原为3-芳基吗啉
通过Petasis borono-Mannich反应的一种变体,可以从芳基硼酸,含水乙二醛和1,2-氨基乙醇轻松制备2-羟基-3-芳基吗啉。我们现在表明,可以通过用甲磺酸酐和三乙胺处理将2-羟基-3-芳基吗啉脱氧为3-芳基吗啉,以提供中间体3,4-二氢-2 H -1,4-恶嗪,然后与用三乙酰氧基硼氢化盐和乙酸制得3-芳基吗啉。该反应序列由容易获得的起始材料(1,2-氨基乙醇,芳基硼酸和乙二醛)组成的三步“两锅”制备3-芳基吗啉,具有优异的官能团耐受性和规模适应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号