当前位置:
X-MOL 学术
›
J. Biotechnol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biotechnologically produced fucosylated oligosaccharides inhibit the binding of human noroviruses to their natural receptors.
Journal of Biotechnology ( IF 4.1 ) Pub Date : 2020-05-05 , DOI: 10.1016/j.jbiotec.2020.05.001 Sami M Derya 1 , Holger Spiegel 2 , Franz-Georg Hanisch 3 , Vasily Morozov 4 , Horst Schroten 4 , Stefan Jennewein 5 , Katja Parschat 1
Journal of Biotechnology ( IF 4.1 ) Pub Date : 2020-05-05 , DOI: 10.1016/j.jbiotec.2020.05.001 Sami M Derya 1 , Holger Spiegel 2 , Franz-Georg Hanisch 3 , Vasily Morozov 4 , Horst Schroten 4 , Stefan Jennewein 5 , Katja Parschat 1
Affiliation
|
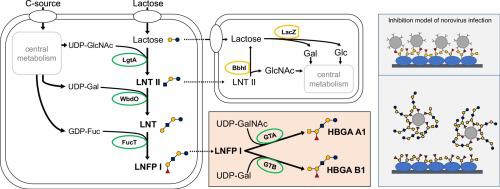
Norovirus infections cause severe gastroenteritis in millions of people every year. Infection requires the recognition of histo-blood group antigens (HBGAs), but such interactions can be inhibited by human milk oligosaccharides (HMOs), which act as structurally-similar decoys. HMO supplements could help to prevent norovirus infections, but the industrial production of complex HMOs is challenging. Here we describe a large-scale fermentation process that yields several kilograms of lacto-N-fucopentaose I (LNFP I). The product was synthesized in Escherichia coli BL21(DE3) cells expressing a recombinant N-acetylglucosaminyltransferase, β(1,3)galactosyltransferase and α(1,2)fucosyltransferase. Subsequent in vitro enzymatic conversion produced HBGA types A1 and B1 for norovirus inhibition assays. These carbohydrates inhibited the binding of GII.17 virus-like particles (VLPs) to type A1 and B1 trisaccharides more efficiently than simpler fucosylated HMOs, which were in turn more effective than any non-fucosylated structures. However, we found that the simpler fucosylated HMOs were more effective than complex molecules such as LNFP I when inhibiting the binding of GII.17 and GII.4 VLPs to human gastric mucins and mucins from human amniotic fluid. Our results show that complex fucosylated HMOs can be produced by large-scale fermentation and that a combination of simple and complex fucosylated structures is more likely to prevent norovirus infections.
中文翻译:

生物技术生产的岩藻糖基化寡糖抑制人诺如病毒与其天然受体的结合。
每年,诺如病毒感染会导致数百万人患上严重的胃肠炎。感染需要识别组织血型抗原(HBGA),但这种相互作用可以被人乳寡糖(HMO)抑制,后者是结构类似的诱饵。HMO补充剂可以帮助预防诺如病毒感染,但是复杂HMO的工业化生产具有挑战性。在这里,我们描述了一个大规模的发酵过程,该过程可产生数公斤的乳酸-N-岩藻糖I(LNFP I)。该产物在表达重组N-乙酰氨基葡萄糖氨基转移酶,β(1,3)半乳糖基转移酶和α(1,2)岩藻糖基转移酶的大肠杆菌BL21(DE3)细胞中合成。随后的体外酶促转化产生了用于诺如病毒抑制试验的HBGA类型A1和B1。这些碳水化合物抑制了GII的结合。与简单的岩藻糖基化的HMO相比,17种病毒样颗粒(VLP)能够更有效地分离A1型和B1型三糖,后者比任何非岩藻糖基化的结构都更有效。但是,我们发现,当抑制GII.17和GII.4 VLP与人胃粘蛋白和人羊水粘蛋白的结合时,较简单的岩藻糖基化HMO比LNFP I等复杂分子更有效。我们的结果表明,复杂的岩藻糖基化HMO可以通过大规模发酵生产,简单和复杂的岩藻糖基化结构的结合更可能预防诺如病毒感染。我们发现,当抑制GII.17和GII.4 VLP与人胃粘蛋白和人羊水粘蛋白的结合时,较简单的岩藻糖基化HMO比LNFP I等复杂分子更有效。我们的结果表明,复杂的岩藻糖基化HMO可以通过大规模发酵生产,简单和复杂的岩藻糖基化结构的结合更可能预防诺如病毒感染。我们发现,当抑制GII.17和GII.4 VLP与人胃粘蛋白和人羊水粘蛋白的结合时,较简单的岩藻糖基化HMO比LNFP I等复杂分子更有效。我们的结果表明,复杂的岩藻糖基化HMO可以通过大规模发酵生产,简单和复杂的岩藻糖基化结构的结合更可能预防诺如病毒感染。
更新日期:2020-05-06
中文翻译:

生物技术生产的岩藻糖基化寡糖抑制人诺如病毒与其天然受体的结合。
每年,诺如病毒感染会导致数百万人患上严重的胃肠炎。感染需要识别组织血型抗原(HBGA),但这种相互作用可以被人乳寡糖(HMO)抑制,后者是结构类似的诱饵。HMO补充剂可以帮助预防诺如病毒感染,但是复杂HMO的工业化生产具有挑战性。在这里,我们描述了一个大规模的发酵过程,该过程可产生数公斤的乳酸-N-岩藻糖I(LNFP I)。该产物在表达重组N-乙酰氨基葡萄糖氨基转移酶,β(1,3)半乳糖基转移酶和α(1,2)岩藻糖基转移酶的大肠杆菌BL21(DE3)细胞中合成。随后的体外酶促转化产生了用于诺如病毒抑制试验的HBGA类型A1和B1。这些碳水化合物抑制了GII的结合。与简单的岩藻糖基化的HMO相比,17种病毒样颗粒(VLP)能够更有效地分离A1型和B1型三糖,后者比任何非岩藻糖基化的结构都更有效。但是,我们发现,当抑制GII.17和GII.4 VLP与人胃粘蛋白和人羊水粘蛋白的结合时,较简单的岩藻糖基化HMO比LNFP I等复杂分子更有效。我们的结果表明,复杂的岩藻糖基化HMO可以通过大规模发酵生产,简单和复杂的岩藻糖基化结构的结合更可能预防诺如病毒感染。我们发现,当抑制GII.17和GII.4 VLP与人胃粘蛋白和人羊水粘蛋白的结合时,较简单的岩藻糖基化HMO比LNFP I等复杂分子更有效。我们的结果表明,复杂的岩藻糖基化HMO可以通过大规模发酵生产,简单和复杂的岩藻糖基化结构的结合更可能预防诺如病毒感染。我们发现,当抑制GII.17和GII.4 VLP与人胃粘蛋白和人羊水粘蛋白的结合时,较简单的岩藻糖基化HMO比LNFP I等复杂分子更有效。我们的结果表明,复杂的岩藻糖基化HMO可以通过大规模发酵生产,简单和复杂的岩藻糖基化结构的结合更可能预防诺如病毒感染。











































 京公网安备 11010802027423号
京公网安备 11010802027423号