Colloid and Interface Science Communications ( IF 4.7 ) Pub Date : 2020-05-06 , DOI: 10.1016/j.colcom.2020.100263 Sharf Ilahi Siddiqui , Prakash Narayan Singh , Nusrat Tara , Shaili Pal , Saif Ali Chaudhry , Indrajit Sinha
|
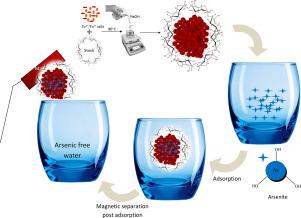
Arsenic is a highly toxic element present in water which needs to be removed from the water to eliminate its adverse health effects on human beings. But the concentration in most natural sources of water is deficient. Therefore investigations for its elimination from dilute aqueous solutions were carried out for obtaining potable water. Fabrication of As(III) specific magnetically recyclable stable nano-adsorbents would be an effective way of solving this tricky problem. The present study investigated the As(III) removal from low concentration aqueous solutions by magnetically recyclable superparamagnetic starch functionalized maghemite nano-adsorbents. For the sake of comparison, arsenic removal from its dilute aqueous solution was carried using both starch functionalized and non-functionalized maghemite nanoparticles as adsorbents. Functionalization by starch led to a significant increase in the As(III) adsorption capacity of the maghemite. The data obtained for arsenite adsorption was found to fit the Freundlich isotherm model, while the kinetics of the adsorption followed the pseudo-second-order model. The maximum Langmuir adsorption capacities achieved for virgin maghemite and functionalized maghemite were 7.14, 7.46, and 7.35 mg/g, and 8.57, 8.88, and 8.67 mg/g at 27, 35 and 45 °C, respectively. The negative value of ∆G (−7.13, −7.21, and −7.50 kJ/mol, for γ-Fe2O3; and −5.50, −5.45 and −5.98 kJ/mol for γ-Fe2O3@starch, at 27, 35 and 45 °C, respectively) indicated the feasibility and spontaneity of the As(III) adsorption on both adsorbents. The negative values of ∆H (−0.0016 kJ/mol for γ-Fe2O3; and −0.00022 kJ/mol for γ-Fe2O3@starch) showed that the adsorption process was exothermic.
中文翻译:

淀粉官能化磁赤铁矿纳米吸附剂从水中去除砷的热力学和动力学研究
砷是水中存在的剧毒元素,需要将其从水中去除,以消除其对人体的不利健康影响。但是大多数自然水源中的浓度不足。因此,进行了从稀水溶液中消除水的研究,以获得饮用水。As(III)特定的可磁循环稳定的纳米吸附剂的制备将是解决这一棘手问题的有效方法。本研究研究了可磁循环的超顺磁性淀粉官能化磁赤铁矿纳米吸附剂从低浓度水溶液中去除As(III)的能力。为了进行比较,使用淀粉官能化的和非官能化的磁赤铁矿纳米粒子作为吸附剂,从稀水溶液中去除砷。淀粉的功能化导致磁赤铁矿的As(III)吸附能力显着提高。发现获得的砷吸附数据符合Freundlich等温模型,而吸附动力学遵循伪二级模型。在27、35和45°C下,纯磁赤铁矿和功能化磁赤铁矿的最大Langmuir吸附容量分别为7.14、7.46和7.35 mg / g,以及8.57、8.88和8.67 mg / g。对于γ-Fe,ΔG的负值(−7.13,−7.21和−7.50 kJ / mol 在27、35和45°C下,纯磁赤铁矿和功能化磁赤铁矿的最大Langmuir吸附容量分别为7.14、7.46和7.35 mg / g,以及8.57、8.88和8.67 mg / g。对于γ-Fe,ΔG的负值(−7.13,−7.21和−7.50 kJ / mol 在27、35和45°C下,纯磁赤铁矿和功能化磁赤铁矿的最大Langmuir吸附容量分别为7.14、7.46和7.35 mg / g,以及8.57、8.88和8.67 mg / g。对于γ-Fe,ΔG的负值(−7.13,−7.21和−7.50 kJ / mol2 O 3 ; 和-5.50,-5.45和-5.98千焦/摩尔为了γ-Fe 2 ö 3 @starch,在27,35和45°C,分别地)指示的作为在两个吸附剂(III)的吸附的可行性和自发性。(对于γ-铁-0.0016千焦/摩尔ΔH的负值2 ö 3 ;和-0.00022千焦/摩尔为了γ-Fe 2 ö 3 @starch)表明,吸附过程是放热的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号