当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery and SAR of Natural-Product-Inspired RXR Agonists with Heterodimer Selectivity to PPARδ-RXR.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-05-06 , DOI: 10.1021/acschembio.0c00146 Ken-Ichi Nakashima 1 , Eiji Yamaguchi , Chihaya Noritake 1 , Yukari Mitsugi , Mayuki Goto , Takao Hirai 1 , Naohito Abe , Eiji Sakai , Masayoshi Oyama , Akichika Itoh , Makoto Inoue 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-05-06 , DOI: 10.1021/acschembio.0c00146 Ken-Ichi Nakashima 1 , Eiji Yamaguchi , Chihaya Noritake 1 , Yukari Mitsugi , Mayuki Goto , Takao Hirai 1 , Naohito Abe , Eiji Sakai , Masayoshi Oyama , Akichika Itoh , Makoto Inoue 1
Affiliation
|
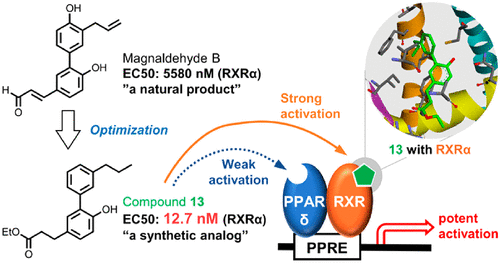
A known natural product, magnaldehyde B, was identified as an agonist of retinoid X receptor (RXR) α. Magnaldehyde B was isolated from Magnolia obovata (Magnoliaceae) and synthesized along with more potent analogs for screening of their RXRα agonistic activities. Structural optimization of magnaldehyde B resulted in the development of a candidate molecule that displayed a 440-fold increase in potency. Receptor–ligand docking simulations indicated that this molecule has the highest affinity with the ligand binding domain of RXRα among the analogs synthesized in this study. Furthermore, the selective activation of the peroxisome proliferator-activated receptor (PPAR) δ-RXR heterodimer with a stronger efficacy compared to those of PPARα-RXR and PPARγ-RXR was achieved in luciferase reporter assays using the PPAR response element driven reporter (PPRE-Luc). The PPARδ activity of the molecule was significantly inhibited by the antagonists of both RXR and PPARδ, whereas the activity of GW501516 was not affected by the RXR antagonist. Furthermore, the molecule exhibited a particularly weak PPARδ agonistic activity in reporter gene assays using the Gal4 hybrid system. The obtained data therefore suggest that the weak PPARδ agonistic activity of the optimized molecule is synergistically enhanced by its own RXR agonistic activity, indicating the potent agonistic activity of the PPARδ-RXR heterodimer.
中文翻译:

对PPARδ-RXR具有异二聚体选择性的自然产物启发的RXR激动剂的发现和合成孔径雷达。
已知的天然产物,甲醛B被确定为类维生素X受体(RXR)α的激动剂。从厚朴木兰中分离出甲醛B(木兰科),并与更有效的类似物合成以筛选其RXRα激动活性。氧化甲醛B的结构优化导致候选分子的开发,该分子的效能提高了440倍。受体-配体对接模拟表明,在该研究中合成的类似物中,该分子与RXRα的配体结合域具有最高的亲和力。此外,在萤光素酶报告基因分析中,使用PPAR响应元件驱动的报告基因(PPRE-卢克)。RXR和PPARδ的拮抗剂均显着抑制了分子的PPARδ活性,而GW501516的活性不受RXR拮抗剂的影响。此外,在使用Gal4杂交系统的报告基因分析中,该分子表现出特别弱的PPARδ激动活性。因此,获得的数据表明,优化分子的弱PPARδ激动活性通过其自身的RXR激动活性协同增强,表明PPARδ-RXR异二聚体的有效激动活性。
更新日期:2020-06-19
中文翻译:

对PPARδ-RXR具有异二聚体选择性的自然产物启发的RXR激动剂的发现和合成孔径雷达。
已知的天然产物,甲醛B被确定为类维生素X受体(RXR)α的激动剂。从厚朴木兰中分离出甲醛B(木兰科),并与更有效的类似物合成以筛选其RXRα激动活性。氧化甲醛B的结构优化导致候选分子的开发,该分子的效能提高了440倍。受体-配体对接模拟表明,在该研究中合成的类似物中,该分子与RXRα的配体结合域具有最高的亲和力。此外,在萤光素酶报告基因分析中,使用PPAR响应元件驱动的报告基因(PPRE-卢克)。RXR和PPARδ的拮抗剂均显着抑制了分子的PPARδ活性,而GW501516的活性不受RXR拮抗剂的影响。此外,在使用Gal4杂交系统的报告基因分析中,该分子表现出特别弱的PPARδ激动活性。因此,获得的数据表明,优化分子的弱PPARδ激动活性通过其自身的RXR激动活性协同增强,表明PPARδ-RXR异二聚体的有效激动活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号