当前位置:
X-MOL 学术
›
Acta Cryst. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
7-Iodo-5-aza-7-deazaguanine ribonucleoside: crystal structure, physical properties, base-pair stability and functionalization.
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2020-04-29 , DOI: 10.1107/s2053229620004684 Dasharath Kondhare 1 , Simone Budow-Busse 1 , Constantin Daniliuc 2 , Frank Seela 1
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2020-04-29 , DOI: 10.1107/s2053229620004684 Dasharath Kondhare 1 , Simone Budow-Busse 1 , Constantin Daniliuc 2 , Frank Seela 1
Affiliation
|
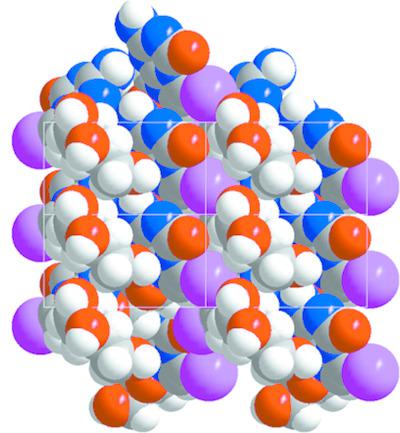
The positional change of nitrogen-7 of the RNA constituent guanosine to the bridgehead position-5 leads to the base-modified nucleoside 5-aza-7-deazaguanosine. Contrary to guanosine, this molecule cannot form Hoogsteen base pairs and the Watson-Crick proton donor site N3-H becomes a proton-acceptor site. This causes changes in nucleobase recognition in nucleic acids and has been used to construct stable `all-purine' DNA and DNA with silver-mediated base pairs. The present work reports the single-crystal X-ray structure of 7-iodo-5-aza-7-deazaguanosine, C10H12IN5O5 (1). The iodinated nucleoside shows an anti conformation at the glycosylic bond and an N conformation (O4'-endo) for the ribose moiety, with an antiperiplanar orientation of the 5'-hydroxy group. Crystal packing is controlled by interactions between nucleobase and sugar moieties. The 7-iodo substituent forms a contact to oxygen-2' of the ribose moiety. Self-pairing of the nucleobases does not take place. A Hirshfeld surface analysis of 1 highlights the contacts of the nucleobase and sugar moiety (O-H...O and N-H...O). The concept of pK-value differences to evaluate base-pair stability was applied to purine-purine base pairing and stable base pairs were predicted for the construction of `all-purine' RNA. Furthermore, the 7-iodo substituent of 1 was functionalized with benzofuran to detect motional constraints by fluorescence spectroscopy.
中文翻译:

7-Iodo-5-aza-7-deazaguanine ribonucleoside:晶体结构、物理性质、碱基对稳定性和功能化。
RNA 成分鸟苷的氮 7 位置改变到桥头位置 5,产生碱基修饰的核苷 5-aza-7-deazaguanosine。与鸟苷相反,该分子不能形成 Hoogsteen 碱基对,并且 Watson-Crick 质子供体位点 N3-H 成为质子受体位点。这会导致核酸中核碱基识别的变化,并已被用于构建稳定的“全嘌呤”DNA 和具有银介导的碱基对的 DNA。目前的工作报告了 7-iodo-5-aza-7-deazaguanosine, C10H12IN5O5 的单晶 X 射线结构 (1)。碘化核苷在糖基键上显示出反构象,在核糖部分上显示出 N 构象(O4'-内),并具有 5'-羟基的反周面方向。晶体堆积由核碱基和糖部分之间的相互作用控制。 7-碘取代基与核糖部分的氧-2'形成接触。核碱基的自我配对不会发生。 1 的 Hirshfeld 表面分析突出显示了核碱基和糖部分(OH...O 和 NH...O)的接触。将评估碱基对稳定性的 pK 值差异概念应用于嘌呤-嘌呤碱基配对,并预测稳定碱基对以构建“全嘌呤”RNA。此外,1 的 7-碘取代基用苯并呋喃功能化,以通过荧光光谱检测运动约束。
更新日期:2020-04-29
中文翻译:

7-Iodo-5-aza-7-deazaguanine ribonucleoside:晶体结构、物理性质、碱基对稳定性和功能化。
RNA 成分鸟苷的氮 7 位置改变到桥头位置 5,产生碱基修饰的核苷 5-aza-7-deazaguanosine。与鸟苷相反,该分子不能形成 Hoogsteen 碱基对,并且 Watson-Crick 质子供体位点 N3-H 成为质子受体位点。这会导致核酸中核碱基识别的变化,并已被用于构建稳定的“全嘌呤”DNA 和具有银介导的碱基对的 DNA。目前的工作报告了 7-iodo-5-aza-7-deazaguanosine, C10H12IN5O5 的单晶 X 射线结构 (1)。碘化核苷在糖基键上显示出反构象,在核糖部分上显示出 N 构象(O4'-内),并具有 5'-羟基的反周面方向。晶体堆积由核碱基和糖部分之间的相互作用控制。 7-碘取代基与核糖部分的氧-2'形成接触。核碱基的自我配对不会发生。 1 的 Hirshfeld 表面分析突出显示了核碱基和糖部分(OH...O 和 NH...O)的接触。将评估碱基对稳定性的 pK 值差异概念应用于嘌呤-嘌呤碱基配对,并预测稳定碱基对以构建“全嘌呤”RNA。此外,1 的 7-碘取代基用苯并呋喃功能化,以通过荧光光谱检测运动约束。











































 京公网安备 11010802027423号
京公网安备 11010802027423号