当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal structure of the Enterococcus faecalis α‐ N ‐acetylgalactosaminidase, a member of the glycoside hydrolase family 31
FEBS Letters ( IF 3.0 ) Pub Date : 2020-05-22 , DOI: 10.1002/1873-3468.13804 Takatsugu Miyazaki 1 , Enoch Y Park 1
FEBS Letters ( IF 3.0 ) Pub Date : 2020-05-22 , DOI: 10.1002/1873-3468.13804 Takatsugu Miyazaki 1 , Enoch Y Park 1
Affiliation
|
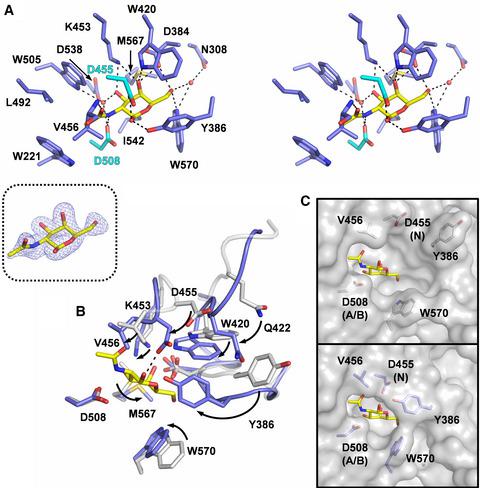
Glycoside hydrolases catalyze the hydrolysis of glycosidic linkages in carbohydrates. The glycoside hydrolase family 31 (GH31) contains α‐glucosidase, α‐xylosidase, α‐galactosidase, and α‐transglycosylase. Recent work has expanded the diversity of substrate specificity of GH31 enzymes, and α‐N‐acetylgalactosaminidases (αGalNAcases) belonging to GH31 have been identified in human gut bacteria. Here, we determined the first crystal structure of a truncated form of GH31 αGalNAcase from the human gut bacterium Enterococcus faecalis. The enzyme has a similar fold to other reported GH31 enzymes and an additional fibronectin type 3‐like domain. Additionally, the structure in complex with N‐acetylgalactosamine reveals that conformations of the active site residues, including its catalytic nucleophile, change to recognize the ligand. Our structural analysis provides insight into the substrate recognition and catalytic mechanism of GH31 αGalNAcases.
中文翻译:

粪肠球菌α-N-乙酰氨基半乳糖苷酶的晶体结构,属于糖苷水解酶家族31
糖苷水解酶催化碳水化合物中糖苷键的水解。糖苷水解酶家族 31 (GH31) 包含 α-葡萄糖苷酶、α-木糖苷酶、α-半乳糖苷酶和α-转糖基酶。最近的工作扩展了 GH31 酶底物特异性的多样性,并且已经在人类肠道细菌中鉴定了属于 GH31 的 α-N-乙酰半乳糖胺酶(αGalNAcases)。在这里,我们确定了来自人类肠道细菌粪肠球菌的截短形式的 GH31 αGalNAcase 的第一个晶体结构。该酶与其他报道的 GH31 酶具有相似的倍数和一个额外的纤连蛋白 3 型结构域。此外,与 N-乙酰半乳糖胺复合的结构表明,活性位点残基的构象,包括其催化亲核试剂,会发生变化以识别配体。
更新日期:2020-05-22
中文翻译:

粪肠球菌α-N-乙酰氨基半乳糖苷酶的晶体结构,属于糖苷水解酶家族31
糖苷水解酶催化碳水化合物中糖苷键的水解。糖苷水解酶家族 31 (GH31) 包含 α-葡萄糖苷酶、α-木糖苷酶、α-半乳糖苷酶和α-转糖基酶。最近的工作扩展了 GH31 酶底物特异性的多样性,并且已经在人类肠道细菌中鉴定了属于 GH31 的 α-N-乙酰半乳糖胺酶(αGalNAcases)。在这里,我们确定了来自人类肠道细菌粪肠球菌的截短形式的 GH31 αGalNAcase 的第一个晶体结构。该酶与其他报道的 GH31 酶具有相似的倍数和一个额外的纤连蛋白 3 型结构域。此外,与 N-乙酰半乳糖胺复合的结构表明,活性位点残基的构象,包括其催化亲核试剂,会发生变化以识别配体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号