当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lipopolysaccharide activates microglia via neuraminidase 1 desialylation of Toll-like Receptor 4.
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-04-12 , DOI: 10.1111/jnc.15024 David Hans Allendorf 1 , Elske Helena Franssen 2 , Guy Charles Brown 1
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-04-12 , DOI: 10.1111/jnc.15024 David Hans Allendorf 1 , Elske Helena Franssen 2 , Guy Charles Brown 1
Affiliation
|
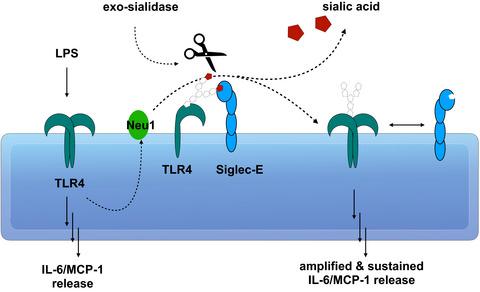
Most cell surface receptors are sialylated, i.e. have sialic acid as the terminal residue of their sugar chains, but can be desialylated by sialidases, such as neuraminidase 1 (Neu1). Desialylation by Neu1 can activate immune cells, such as neutrophils, macrophages and monocytes. We investigated the role of Neu1 in activation of microglia using BV‐2 cells (a murine microglial cell line) by cytokine ELISAs, enzyme activity assays, antibody/lectin binding and proximity labelling. We found that lipopolysaccharide (LPS) activation caused an increase in Neu1 protein on the cell surface, and an increase in surface sialidase activity that was prevented by Neu1 knockdown. Moreover, LPS induced interleukin 6 (IL‐6) and MCP‐1 release, which was reduced by Neu1 knockdown and increased by Neu1 over‐expression. Neu1 knockdown also prevented the maintenance of IL‐6 release by microglia after LPS was removed. Sialidase treatment of the cells was sufficient to induce IL‐6 release, prevented by inhibiting toll‐like receptor 4 (TLR4). Neu1 was found in close proximity to TLR4 on the surface of cells, and LPS induced desialylation of TLR4 on the cell surface, prevented by Neu1 knockdown. Sialic acid‐binding immunoglobulin‐like lectin E was found to bind to TLR4 via sialic acid residues and inhibit IL‐6 release by BV‐2 cells. We conclude that LPS causes Neu1 to translocate to the cell surface, where it desialylates TLR4, releasing inhibitory sialic acid‐binding immunoglobulin‐like lectin E, enhancing and maintaining inflammatory activation of the microglia. Thus, sialylation is a potent regulator of microglial activation, and Neu1 may be a target to reduce activation of microglia.
中文翻译:

脂多糖通过神经氨酸酶1对Toll样受体4的去唾液酸化作用激活小胶质细胞。
大多数细胞表面受体都被唾液酸化,即以唾液酸作为糖链的末端残基,但是可以被唾液酸化酶(例如神经氨酸酶1(Neu1))去唾液酸化。Neu1进行的脱唾液酸化可以激活免疫细胞,例如嗜中性粒细胞,巨噬细胞和单核细胞。我们通过细胞因子ELISA,酶活性测定,抗体/凝集素结合和邻近标记,研究了Neu1在BV-2细胞(鼠小胶质细胞系)激活小胶质细胞中的作用。我们发现脂多糖(LPS)激活导致细胞表面Neu1蛋白的增加,以及由Neu1敲低阻止的表面唾液酸酶活性的增加。此外,LPS诱导白介素6(IL-6)和MCP-1释放,这通过Neu1敲低而降低,而由于Neu1过表达而增加。去除LPS后,Neu1敲低还阻止了小胶质细胞维持IL-6的释放。细胞的唾液酸酶处理足以诱导IL-6释放,这是通过抑制Toll样受体4(TLR4)阻止的。发现Neu1紧邻细胞表面上的TLR4,LPS诱导了细胞表面TLR4的去唾液酸化,这被Neu1敲低阻止了。发现唾液酸结合免疫球蛋白样凝集素E通过唾液酸残基与TLR4结合并抑制BV-2细胞释放IL-6。我们得出的结论是,LPS导致Neu1易位至细胞表面,在其中将TLR4脱唾液酸化,释放出抑制性唾液酸结合免疫球蛋白样凝集素E,增强并维持小胶质细胞的炎症激活。因此,唾液酸化是小胶质细胞激活的有效调节剂,
更新日期:2020-04-12
中文翻译:

脂多糖通过神经氨酸酶1对Toll样受体4的去唾液酸化作用激活小胶质细胞。
大多数细胞表面受体都被唾液酸化,即以唾液酸作为糖链的末端残基,但是可以被唾液酸化酶(例如神经氨酸酶1(Neu1))去唾液酸化。Neu1进行的脱唾液酸化可以激活免疫细胞,例如嗜中性粒细胞,巨噬细胞和单核细胞。我们通过细胞因子ELISA,酶活性测定,抗体/凝集素结合和邻近标记,研究了Neu1在BV-2细胞(鼠小胶质细胞系)激活小胶质细胞中的作用。我们发现脂多糖(LPS)激活导致细胞表面Neu1蛋白的增加,以及由Neu1敲低阻止的表面唾液酸酶活性的增加。此外,LPS诱导白介素6(IL-6)和MCP-1释放,这通过Neu1敲低而降低,而由于Neu1过表达而增加。去除LPS后,Neu1敲低还阻止了小胶质细胞维持IL-6的释放。细胞的唾液酸酶处理足以诱导IL-6释放,这是通过抑制Toll样受体4(TLR4)阻止的。发现Neu1紧邻细胞表面上的TLR4,LPS诱导了细胞表面TLR4的去唾液酸化,这被Neu1敲低阻止了。发现唾液酸结合免疫球蛋白样凝集素E通过唾液酸残基与TLR4结合并抑制BV-2细胞释放IL-6。我们得出的结论是,LPS导致Neu1易位至细胞表面,在其中将TLR4脱唾液酸化,释放出抑制性唾液酸结合免疫球蛋白样凝集素E,增强并维持小胶质细胞的炎症激活。因此,唾液酸化是小胶质细胞激活的有效调节剂,











































 京公网安备 11010802027423号
京公网安备 11010802027423号