当前位置:
X-MOL 学术
›
Photochem. Photobiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Why are the elemental nonmetals (F2 , Cl2 , Br2 , I2 , S8 , P4 ) of so many hues or of any hues and where is the chromophore? Insight into intera-X-X bonds
Photochemistry and Photobiology ( IF 2.6 ) Pub Date : 2020-05-04 , DOI: 10.1111/php.13270 Shakeela Jabeen 1, 2 , Alexander Greer 1, 2 , Kathleen F Edwards 3 , Joel F Liebman 4
Photochemistry and Photobiology ( IF 2.6 ) Pub Date : 2020-05-04 , DOI: 10.1111/php.13270 Shakeela Jabeen 1, 2 , Alexander Greer 1, 2 , Kathleen F Edwards 3 , Joel F Liebman 4
Affiliation
|
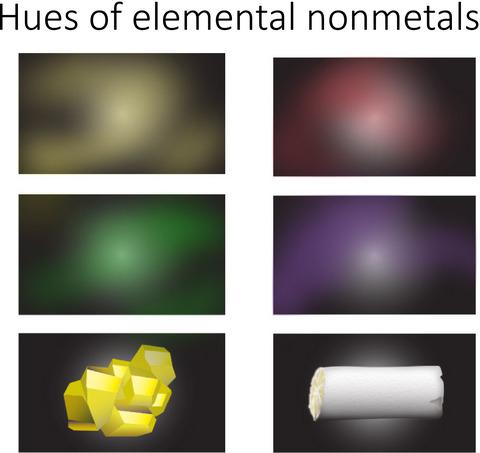
A unique approach is used to relate the HOMO‐LUMO energy difference to the difference between the ionization potential (IP) and electron affinity (EA) to assist in deducing not only the colors, but also chromophores in elemental nonmetals. Our analysis focuses on compounds with lone pair electrons and σ electrons, namely X2 (X = F, Cl, Br, I), S8 and P4. For the dihalogens, the [IP – EA] energies are found to be: F2 (12.58 eV), Cl2 (8.98 eV), Br2 (7.90 eV), I2 (6.78 eV). We suggest that the interahalogen X–X bond itself is the chromophore for these dihalogens, in which the light absorbed by the F2, Cl2, Br2, I2 leads to longer wavelengths in the visible by a π → σ* transition. Trace impurities are a likely case of cyclic S8 which contains amounts of selenium leading to a yellow color, where the [IP – EA] energy of S8 is found to be 7.02 eV. Elemental P4 with an [IP – EA] energy of 9.09 eV contains a tetrahedral and σ aromatic structure. In future work, refinement of the analysis will be required for compounds with π electrons and σ electrons, such as polycyclic aromatic hydrocarbons (PAHs).
中文翻译:

为什么非金属元素(F2、Cl2、Br2、I2、S8、P4)有这么多颜色或任何颜色,生色团在哪里?深入了解 XX 间债券
使用一种独特的方法将 HOMO-LUMO 能量差异与电离势 (IP) 和电子亲和力 (EA) 之间的差异联系起来,不仅有助于推断颜色,还有助于推断元素非金属中的生色团。我们的分析侧重于具有孤对电子和 σ 电子的化合物,即 X2 (X = F、Cl、Br、I)、S8 和 P4。对于二卤素,发现 [IP – EA] 能量为:F2 (12.58 eV)、Cl2 (8.98 eV)、Br2 (7.90 eV)、I2 (6.78 eV)。我们建议卤素间 X-X 键本身是这些二卤化物的发色团,其中 F2、Cl2、Br2、I2 吸收的光通过 π → σ* 跃迁导致可见光中更长的波长。微量杂质很可能是环状 S8,其中含有大量硒,导致黄色,其中 S8 的 [IP – EA] 能量为 7.02 eV。[IP – EA] 能量为 9.09 eV 的 P4 元素包含四面体和 σ 芳族结构。在未来的工作中,需要对具有 π 电子和 σ 电子的化合物(例如多环芳烃 (PAH))进行分析。
更新日期:2020-05-04
中文翻译:

为什么非金属元素(F2、Cl2、Br2、I2、S8、P4)有这么多颜色或任何颜色,生色团在哪里?深入了解 XX 间债券
使用一种独特的方法将 HOMO-LUMO 能量差异与电离势 (IP) 和电子亲和力 (EA) 之间的差异联系起来,不仅有助于推断颜色,还有助于推断元素非金属中的生色团。我们的分析侧重于具有孤对电子和 σ 电子的化合物,即 X2 (X = F、Cl、Br、I)、S8 和 P4。对于二卤素,发现 [IP – EA] 能量为:F2 (12.58 eV)、Cl2 (8.98 eV)、Br2 (7.90 eV)、I2 (6.78 eV)。我们建议卤素间 X-X 键本身是这些二卤化物的发色团,其中 F2、Cl2、Br2、I2 吸收的光通过 π → σ* 跃迁导致可见光中更长的波长。微量杂质很可能是环状 S8,其中含有大量硒,导致黄色,其中 S8 的 [IP – EA] 能量为 7.02 eV。[IP – EA] 能量为 9.09 eV 的 P4 元素包含四面体和 σ 芳族结构。在未来的工作中,需要对具有 π 电子和 σ 电子的化合物(例如多环芳烃 (PAH))进行分析。











































 京公网安备 11010802027423号
京公网安备 11010802027423号