当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystal structure of the nucleoid-associated protein Fis (PA4853) from Pseudomonas aeruginosa.
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2020-05-01 , DOI: 10.1107/s2053230x20005427 Juan Zhou 1 , Zengqiang Gao 2 , Heng Zhang 2 , Yuhui Dong 2
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2020-05-01 , DOI: 10.1107/s2053230x20005427 Juan Zhou 1 , Zengqiang Gao 2 , Heng Zhang 2 , Yuhui Dong 2
Affiliation
|
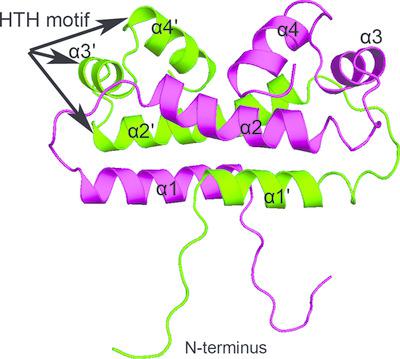
Factor for inversion stimulation (Fis) is a versatile bacterial nucleoid‐associated protein that can directly bind and bend DNA to influence DNA topology. It also plays crucial roles in regulating bacterial virulence factors and in optimizing bacterial adaptation to various environments. Fis from Pseudomonas aeruginosa (PA4853, referred to as PaFis) has recently been found to be required for virulence by regulating the expression of type III secretion system (T3SS) genes. PaFis can specifically bind to the promoter region of exsA, which functions as a T3SS master regulator, to regulate its expression and plays an essential role in transcription elongation from exsB to exsA. Here, the crystal structure of PaFis, which is composed of a four‐helix bundle and forms a homodimer, is reported. PaFis shows remarkable structural similarities to the well studied Escherichia coli Fis (EcFis), including an N‐terminal flexible loop and a C‐terminal helix–turn–helix (HTH) motif. However, the critical residues for Hin‐catalyzed DNA inversion in the N‐terminal loop of EcFis are not conserved in PaFis and further studies are required to investigate its exact role. A gel‐electrophoresis mobility‐shift assay showed that PaFis can efficiently bind to the promoter region of exsA. Structure‐based mutagenesis revealed that several conserved basic residues in the HTH motif play essential roles in DNA binding. These structural and biochemical studies may help in understanding the role of PaFis in the regulation of T3SS expression and in virulence.
中文翻译:

来自铜绿假单胞菌的类核相关蛋白 Fis (PA4853) 的晶体结构。
反转刺激因子 (Fis) 是一种多功能细菌核相关蛋白,可以直接结合和弯曲 DNA 以影响 DNA 拓扑结构。它还在调节细菌毒力因子和优化细菌对各种环境的适应方面发挥着至关重要的作用。最近发现来自铜绿假单胞菌的Fis (PA4853,简称PaFis)通过调节III型分泌系统(T3SS)基因的表达来产生毒力。PaFis 可以特异性结合exsA的启动子区域,作为 T3SS 主调节因子,调节其表达,并在从exsB到exsA的转录延伸中发挥重要作用。在这里,报道了 PaFis 的晶体结构,它由四螺旋束组成并形成同二聚体。PaFis 与经过充分研究的大肠杆菌Fis (EcFis) 显示出显着的结构相似性,包括 N 端柔性环和 C 端螺旋-转角-螺旋 (HTH) 基序。然而,EcFis N 末端环中 Hin 催化 DNA 倒转的关键残基在 PaFis 中并不保守,需要进一步研究来探究其确切作用。凝胶电泳迁移率变动分析表明 PaFis 可以有效结合exsA的启动子区域。基于结构的诱变揭示了 HTH 基序中的几个保守的碱性残基在 DNA 结合中发挥着重要作用。这些结构和生化研究可能有助于了解 PaFis 在 T3SS 表达和毒力调节中的作用。
更新日期:2020-05-01
中文翻译:

来自铜绿假单胞菌的类核相关蛋白 Fis (PA4853) 的晶体结构。
反转刺激因子 (Fis) 是一种多功能细菌核相关蛋白,可以直接结合和弯曲 DNA 以影响 DNA 拓扑结构。它还在调节细菌毒力因子和优化细菌对各种环境的适应方面发挥着至关重要的作用。最近发现来自铜绿假单胞菌的Fis (PA4853,简称PaFis)通过调节III型分泌系统(T3SS)基因的表达来产生毒力。PaFis 可以特异性结合exsA的启动子区域,作为 T3SS 主调节因子,调节其表达,并在从exsB到exsA的转录延伸中发挥重要作用。在这里,报道了 PaFis 的晶体结构,它由四螺旋束组成并形成同二聚体。PaFis 与经过充分研究的大肠杆菌Fis (EcFis) 显示出显着的结构相似性,包括 N 端柔性环和 C 端螺旋-转角-螺旋 (HTH) 基序。然而,EcFis N 末端环中 Hin 催化 DNA 倒转的关键残基在 PaFis 中并不保守,需要进一步研究来探究其确切作用。凝胶电泳迁移率变动分析表明 PaFis 可以有效结合exsA的启动子区域。基于结构的诱变揭示了 HTH 基序中的几个保守的碱性残基在 DNA 结合中发挥着重要作用。这些结构和生化研究可能有助于了解 PaFis 在 T3SS 表达和毒力调节中的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号