当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of the 4-hydroxy-tetrahydrodipicolinate synthase from the thermoacidophilic methanotroph Methylacidiphilum fumariolicum SolV and the phylogeny of the aminotransferase pathway.
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2020-05-01 , DOI: 10.1107/s2053230x20005294 Rob A Schmitz 1 , Andreas Dietl 2 , Melanie Müller 2 , Tom Berben 1 , Huub J M Op den Camp 1 , Thomas R M Barends 2
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2020-05-01 , DOI: 10.1107/s2053230x20005294 Rob A Schmitz 1 , Andreas Dietl 2 , Melanie Müller 2 , Tom Berben 1 , Huub J M Op den Camp 1 , Thomas R M Barends 2
Affiliation
|
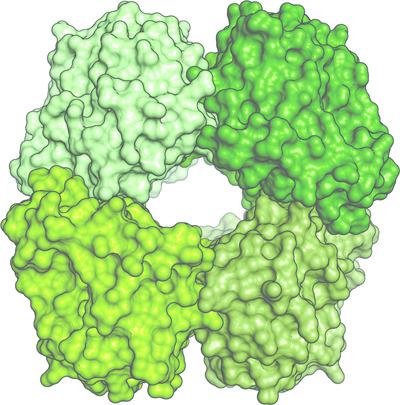
The enzyme 4‐hydroxy‐tetrahydrodipicolinate synthase (DapA) is involved in the production of lysine and precursor molecules for peptidoglycan synthesis. In a multistep reaction, DapA converts pyruvate and l‐aspartate‐4‐semialdehyde to 4‐hydroxy‐2,3,4,5‐tetrahydrodipicolinic acid. In many organisms, lysine binds allosterically to DapA, causing negative feedback, thus making the enzyme an important regulatory component of the pathway. Here, the 2.1 Å resolution crystal structure of DapA from the thermoacidophilic methanotroph Methylacidiphilum fumariolicum SolV is reported. The enzyme crystallized as a contaminant of a protein preparation from native biomass. Genome analysis reveals that M. fumariolicum SolV utilizes the recently discovered aminotransferase pathway for lysine biosynthesis. Phylogenetic analyses of the genes involved in this pathway shed new light on the distribution of this pathway across the three domains of life.
中文翻译:

来自嗜热嗜酸甲烷氧化菌 Mmethylacidiphilum fumariolicum SolV 的 4-羟基-四氢吡啶二羧酸合酶的结构和转氨酶途径的系统发育。
4-羟基四氢吡啶二羧酸合酶 (DapA) 参与赖氨酸和肽聚糖合成前体分子的产生。在多步反应中,DapA 将丙酮酸和l-天冬氨酸-4-半醛转化为 4-羟基-2,3,4,5-四氢吡啶二甲酸。在许多生物体中,赖氨酸以变构方式与 DapA 结合,引起负反馈,从而使该酶成为该途径的重要调节成分。在此,报道了来自嗜热嗜酸甲烷氧化菌Mmethylacidiphilum fumariolicum SolV的 DapA 的 2.1 Å 分辨率晶体结构。该酶作为来自天然生物质的蛋白质制剂的污染物结晶。基因组分析表明,M. fumariolicum SolV 利用最近发现的转氨酶途径进行赖氨酸生物合成。对参与该途径的基因的系统发育分析为该途径在生命的三个领域的分布提供了新的线索。
更新日期:2020-05-01
中文翻译:

来自嗜热嗜酸甲烷氧化菌 Mmethylacidiphilum fumariolicum SolV 的 4-羟基-四氢吡啶二羧酸合酶的结构和转氨酶途径的系统发育。
4-羟基四氢吡啶二羧酸合酶 (DapA) 参与赖氨酸和肽聚糖合成前体分子的产生。在多步反应中,DapA 将丙酮酸和l-天冬氨酸-4-半醛转化为 4-羟基-2,3,4,5-四氢吡啶二甲酸。在许多生物体中,赖氨酸以变构方式与 DapA 结合,引起负反馈,从而使该酶成为该途径的重要调节成分。在此,报道了来自嗜热嗜酸甲烷氧化菌Mmethylacidiphilum fumariolicum SolV的 DapA 的 2.1 Å 分辨率晶体结构。该酶作为来自天然生物质的蛋白质制剂的污染物结晶。基因组分析表明,M. fumariolicum SolV 利用最近发现的转氨酶途径进行赖氨酸生物合成。对参与该途径的基因的系统发育分析为该途径在生命的三个领域的分布提供了新的线索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号