当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of the N-terminal domain of ClpC1 in complex with the antituberculosis natural product ecumicin reveals unique binding interactions.
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-04-23 , DOI: 10.1107/s2059798320004027 Nina M Wolf 1 , Hyun Lee 1 , Daniel Zagal 2 , Joo Won Nam 2 , Dong Chan Oh 3 , Hanki Lee 4 , Joo Won Suh 4 , Guido F Pauli 1 , Sanghyun Cho 1 , Celerino Abad-Zapatero 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-04-23 , DOI: 10.1107/s2059798320004027 Nina M Wolf 1 , Hyun Lee 1 , Daniel Zagal 2 , Joo Won Nam 2 , Dong Chan Oh 3 , Hanki Lee 4 , Joo Won Suh 4 , Guido F Pauli 1 , Sanghyun Cho 1 , Celerino Abad-Zapatero 1
Affiliation
|
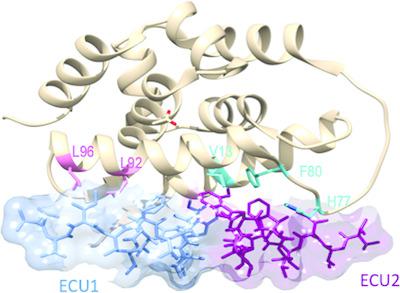
The biological processes related to protein homeostasis in Mycobacterium tuberculosis, the etiologic agent of tuberculosis, have recently been established as critical pathways for therapeutic intervention. Proteins of particular interest are ClpC1 and the ClpC1-ClpP1-ClpP2 proteasome complex. The structure of the potent antituberculosis macrocyclic depsipeptide ecumicin complexed with the N-terminal domain of ClpC1 (ClpC1-NTD) is presented here. Crystals of the ClpC1-NTD-ecumicin complex were monoclinic (unit-cell parameters a = 80.0, b = 130.0, c = 112.0 Å, β = 90.07°; space group P21; 12 complexes per asymmetric unit) and diffracted to 2.5 Å resolution. The structure was solved by molecular replacement using the self-rotation function to resolve space-group ambiguities. The new structure of the ecumicin complex showed a unique 1:2 (target:ligand) stoichiometry exploiting the intramolecular dyad in the α-helical fold of the target N-terminal domain. The structure of the ecumicin complex unveiled extensive interactions in the uniquely extended N-terminus, a critical binding site for the known cyclopeptide complexes. This structure, in comparison with the previously reported rufomycin I complex, revealed unique features that could be relevant for understanding the mechanism of action of these potential antituberculosis drug leads. Comparison of the ecumicin complex and the ClpC1-NTD-L92S/L96P double-mutant structure with the available structures of rufomycin I and cyclomarin A complexes revealed a range of conformational changes available to this small N-terminal helical domain and the minor helical alterations involved in the antibiotic-resistance mechanism. The different modes of binding and structural alterations could be related to distinct modes of action.
中文翻译:

ClpC1的N末端结构域与抗结核天然产物ecumicin的复合结构揭示了独特的结合相互作用。
最近已确定与结核分枝杆菌(结核病的病原体)中蛋白质稳态有关的生物学过程是治疗干预的关键途径。特别感兴趣的蛋白质是ClpC1和ClpC1-ClpP1-ClpP2蛋白酶体复合物。此处介绍了与ClpC1(ClpC1-NTD)N端结构域复合的强效抗结核大环二肽紫杉醇轻铁蛋白的结构。ClpC1-NTD-肌钙蛋白复合物的晶体是单斜晶的(晶胞参数a = 80.0,b = 130.0,c = 112.0Å,β= 90.07°;空间群P21;每个不对称单位12个复合物),衍射至2.5Å分辨率。 。该结构通过使用自转功能解决空间组歧义的分子置换来解决。ecumicin复合物的新结构显示出独特的1:2(目标:配体)的化学计量,利用目标N端结构域的α螺旋折叠中的分子内二分体。岩umi素复合物的结构揭示了在独特延伸的N末端的广泛相互作用,N末端是已知环肽复合物的关键结合位点。与先前报道的红霉素I复合物相比,该结构揭示了独特的功能,可能与理解这些潜在的抗结核药物线索的作用机制有关。将ecumicin复合物和ClpC1-NTD-L92S / L96P双突变体结构与红霉素I和cyclomarin A复合物的可用结构进行比较,发现该N末端小螺旋结构域可利用的构象变化范围和涉及的较小螺旋变化在抗药性机制中。
更新日期:2020-04-23
中文翻译:

ClpC1的N末端结构域与抗结核天然产物ecumicin的复合结构揭示了独特的结合相互作用。
最近已确定与结核分枝杆菌(结核病的病原体)中蛋白质稳态有关的生物学过程是治疗干预的关键途径。特别感兴趣的蛋白质是ClpC1和ClpC1-ClpP1-ClpP2蛋白酶体复合物。此处介绍了与ClpC1(ClpC1-NTD)N端结构域复合的强效抗结核大环二肽紫杉醇轻铁蛋白的结构。ClpC1-NTD-肌钙蛋白复合物的晶体是单斜晶的(晶胞参数a = 80.0,b = 130.0,c = 112.0Å,β= 90.07°;空间群P21;每个不对称单位12个复合物),衍射至2.5Å分辨率。 。该结构通过使用自转功能解决空间组歧义的分子置换来解决。ecumicin复合物的新结构显示出独特的1:2(目标:配体)的化学计量,利用目标N端结构域的α螺旋折叠中的分子内二分体。岩umi素复合物的结构揭示了在独特延伸的N末端的广泛相互作用,N末端是已知环肽复合物的关键结合位点。与先前报道的红霉素I复合物相比,该结构揭示了独特的功能,可能与理解这些潜在的抗结核药物线索的作用机制有关。将ecumicin复合物和ClpC1-NTD-L92S / L96P双突变体结构与红霉素I和cyclomarin A复合物的可用结构进行比较,发现该N末端小螺旋结构域可利用的构象变化范围和涉及的较小螺旋变化在抗药性机制中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号