当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural basis of carbohydrate binding in domain C of a type I pullulanase from Paenibacillus barengoltzii.
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-05-01 , DOI: 10.1107/s205979832000409x Ping Huang 1 , Shiwang Wu 1 , Shaoqing Yang 1 , Qiaojuan Yan 2 , Zhengqiang Jiang 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-05-01 , DOI: 10.1107/s205979832000409x Ping Huang 1 , Shiwang Wu 1 , Shaoqing Yang 1 , Qiaojuan Yan 2 , Zhengqiang Jiang 1
Affiliation
|
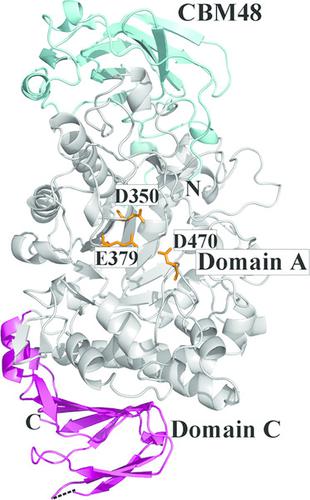
Pullulanase (EC 3.2.1.41) is a well known starch‐debranching enzyme that catalyzes the cleavage of α‐1,6‐glycosidic linkages in α‐glucans such as starch and pullulan. Crystal structures of a type I pullulanase from Paenibacillus barengoltzii (PbPulA) and of PbPulA in complex with maltopentaose (G5), maltohexaose (G6)/α‐cyclodextrin (α‐CD) and β‐cyclodextrin (β‐CD) were determined in order to better understand substrate binding to this enzyme. PbPulA belongs to glycoside hydrolase (GH) family 13 subfamily 14 and is composed of three domains (CBM48, A and C). Three carbohydrate‐binding sites identified in PbPulA were located in CBM48, near the active site and in domain C, respectively. The binding site in CBM48 was specific for β‐CD, while that in domain C has not been reported for other pullulanases. The domain C binding site had higher affinity for α‐CD than for G6; a small motif (FGGEH) seemed to be one of the major determinants for carbohydrate binding in this domain. Structure‐based mutations of several surface‐exposed aromatic residues in CBM48 and domain C had a debilitating effect on the activity of the enzyme. These results suggest that both CBM48 and domain C play a role in binding substrates. The crystal forms described contribute to the understanding of pullulanase domain–carbohydrate interactions.
中文翻译:

糖结合于来自巴氏芽孢杆菌的I型支链淀粉酶的结构域C中的碳水化合物。
支链淀粉酶(EC 3.2.1.41)是一种众所周知的淀粉脱支酶,可催化淀粉和支链淀粉等α-葡聚糖中α-1,6-糖苷键的裂解。确定了来自Bareniolillus Barengoltzii(Pb PulA)和Pb PulA的I型支链淀粉酶与麦芽五糖(G5),麦芽六糖(G6)/α-环糊精(α-CD)和β-环糊精(β-CD)的复合物的晶体结构为了更好地了解底物与该酶的结合。Pb PulA属于糖苷水解酶(GH)家族13亚家族14,由三个域(CBM48,A和C)组成。铅中鉴定出三个碳水化合物结合位点PulA分别位于CBM48中,活性位点附近和结构域C中。CBM48中的结合位点是β-CD特异的,而其他支链淀粉酶的C域中则没有报道。C域结合位点对α-CD的亲和力高于对G6的亲和力。一个小基序(FGGEH)似乎是该域中碳水化合物结合的主要决定因素之一。CBM48和域C中几个表面暴露的芳香族残基的基于结构的突变对酶的活性具有破坏作用。这些结果表明,CBM48和结构域C均在结合底物中起作用。所描述的晶体形式有助于理解支链淀粉酶域与碳水化合物的相互作用。
更新日期:2020-05-01
中文翻译:

糖结合于来自巴氏芽孢杆菌的I型支链淀粉酶的结构域C中的碳水化合物。
支链淀粉酶(EC 3.2.1.41)是一种众所周知的淀粉脱支酶,可催化淀粉和支链淀粉等α-葡聚糖中α-1,6-糖苷键的裂解。确定了来自Bareniolillus Barengoltzii(Pb PulA)和Pb PulA的I型支链淀粉酶与麦芽五糖(G5),麦芽六糖(G6)/α-环糊精(α-CD)和β-环糊精(β-CD)的复合物的晶体结构为了更好地了解底物与该酶的结合。Pb PulA属于糖苷水解酶(GH)家族13亚家族14,由三个域(CBM48,A和C)组成。铅中鉴定出三个碳水化合物结合位点PulA分别位于CBM48中,活性位点附近和结构域C中。CBM48中的结合位点是β-CD特异的,而其他支链淀粉酶的C域中则没有报道。C域结合位点对α-CD的亲和力高于对G6的亲和力。一个小基序(FGGEH)似乎是该域中碳水化合物结合的主要决定因素之一。CBM48和域C中几个表面暴露的芳香族残基的基于结构的突变对酶的活性具有破坏作用。这些结果表明,CBM48和结构域C均在结合底物中起作用。所描述的晶体形式有助于理解支链淀粉酶域与碳水化合物的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号