当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and thermodynamic analyses of interactions between death-associated protein kinase 1 and anthraquinones.
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-05-01 , DOI: 10.1107/s2059798320003940 Takeshi Yokoyama 1 , Peter Wijaya 1 , Yuto Kosaka 1 , Mineyuki Mizuguchi 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-05-01 , DOI: 10.1107/s2059798320003940 Takeshi Yokoyama 1 , Peter Wijaya 1 , Yuto Kosaka 1 , Mineyuki Mizuguchi 1
Affiliation
|
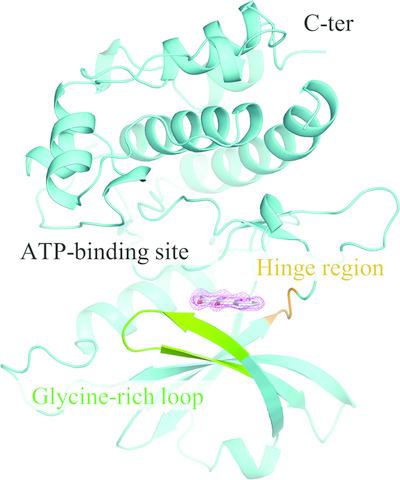
Death‐associated protein kinase 1 (DAPK1) is a serine/threonine protein kinase that regulates apoptosis and autophagy. DAPK1 is considered to be a therapeutic target for amyloid‐β deposition, endometrial adenocarcinomas and acute ischemic stroke. Here, the potent inhibitory activity of the natural anthraquinone purpurin against DAPK1 phosphorylation is shown. Thermodynamic analysis revealed that while the binding affinity of purpurin is similar to that of CPR005231, which is a DAPK1 inhibitor with an imidazopyridazine moiety, the binding of purpurin was more enthalpically favorable. In addition, the inhibition potencies were correlated with the enthalpic changes but not with the binding affinities. Crystallographic analysis of the DAPK1–purpurin complex revealed that the formation of a hydrogen‐bond network is likely to contribute to the favorable enthalpic changes and that stabilization of the glycine‐rich loop may cause less favorable entropic changes. The present findings indicate that purpurin may be a good lead compound for the discovery of inhibitors of DAPK1, and the observation of enthalpic changes could provide important clues for drug development.
中文翻译:

死亡相关蛋白激酶 1 和蒽醌之间相互作用的结构和热力学分析。
死亡相关蛋白激酶 1 (DAPK1) 是一种调节细胞凋亡和自噬的丝氨酸/苏氨酸蛋白激酶。 DAPK1 被认为是β淀粉样蛋白沉积、子宫内膜腺癌和急性缺血性中风的治疗靶点。这里显示了天然蒽醌红紫素对 DAPK1 磷酸化的有效抑制活性。热力学分析表明,虽然红紫素的结合亲和力与 CPR005231(一种带有咪唑并哒嗪部分的 DAPK1 抑制剂)相似,但红紫素的结合在热函上更有利。此外,抑制效力与焓变相关,但与结合亲和力无关。 DAPK1-红紫素复合物的晶体学分析表明,氢键网络的形成可能有助于有利的焓变,而富含甘氨酸的环的稳定可能会导致不太有利的熵变。目前的研究结果表明,红紫素可能是发现 DAPK1 抑制剂的良好先导化合物,而热函变化的观察可以为药物开发提供重要线索。
更新日期:2020-05-01
中文翻译:

死亡相关蛋白激酶 1 和蒽醌之间相互作用的结构和热力学分析。
死亡相关蛋白激酶 1 (DAPK1) 是一种调节细胞凋亡和自噬的丝氨酸/苏氨酸蛋白激酶。 DAPK1 被认为是β淀粉样蛋白沉积、子宫内膜腺癌和急性缺血性中风的治疗靶点。这里显示了天然蒽醌红紫素对 DAPK1 磷酸化的有效抑制活性。热力学分析表明,虽然红紫素的结合亲和力与 CPR005231(一种带有咪唑并哒嗪部分的 DAPK1 抑制剂)相似,但红紫素的结合在热函上更有利。此外,抑制效力与焓变相关,但与结合亲和力无关。 DAPK1-红紫素复合物的晶体学分析表明,氢键网络的形成可能有助于有利的焓变,而富含甘氨酸的环的稳定可能会导致不太有利的熵变。目前的研究结果表明,红紫素可能是发现 DAPK1 抑制剂的良好先导化合物,而热函变化的观察可以为药物开发提供重要线索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号