当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of P46, an immunodominant surface protein from Mycoplasma hyopneumoniae: interaction with a monoclonal antibody.
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-05-01 , DOI: 10.1107/s2059798320003903 Alicia Guasch 1 , Jordi Montané 2 , Alexandra Moros 2 , Jaume Piñol 3 , Marta Sitjà 2 , Luis González-González 2 , Ignasi Fita 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-05-01 , DOI: 10.1107/s2059798320003903 Alicia Guasch 1 , Jordi Montané 2 , Alexandra Moros 2 , Jaume Piñol 3 , Marta Sitjà 2 , Luis González-González 2 , Ignasi Fita 1
Affiliation
|
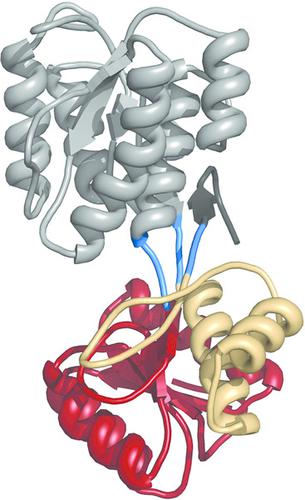
Mycoplasma hyopneumoniae is a prokaryotic pathogen that colonizes the respiratory ciliated epithelial cells in swine. Infected animals suffer respiratory lesions, causing major economic losses in the porcine industry. Characterization of the immunodominant membrane‐associated proteins from M. hyopneumoniae may be instrumental in the development of new therapeutic approaches. Here, the crystal structure of P46, one of the main surface‐antigen proteins, from M. hyopneumoniae is presented and shows N‐ and C‐terminal α/β domains connected by a hinge. The structures solved in this work include a ligand‐free open form of P46 (3.1 Å resolution) and two ligand‐bound structures of P46 with maltose (2.5 Å resolution) and xylose (3.5 Å resolution) in open and closed conformations, respectively. The ligand‐binding site is buried in the cleft between the domains at the hinge region. The two domains of P46 can rotate with respect to each other, giving open or closed alternative conformations. In agreement with this structural information, sequence analyses show similarities to substrate‐binding members of the ABC transporter superfamily, with P46 facing the extracellular side as a functional subunit. In the structure with xylose, P46 was also bound to a high‐affinity (Kd = 29 nM) Fab fragment from a monoclonal antibody, allowing the characterization of a structural epitope in P46 that exclusively involves residues from the C‐terminal domain. The Fab structure in the complex with P46 shows only small conformational rearrangements in the six complementarity‐determining regions (CDRs) with respect to the unbound Fab (the structure of which is also determined in this work at 1.95 Å resolution). The structural information that is now available should contribute to a better understanding of sugar nutrient intake by M. hyopneumoniae. This information will also allow the design of protocols and strategies for the generation of new vaccines against this important swine pathogen.
中文翻译:

P46的结构,猪肺炎支原体的一种免疫优势表面蛋白:与单克隆抗体的相互作用。
猪肺炎支原体是一种原核病原体,定居在猪呼吸道纤毛上皮细胞中。被感染的动物遭受呼吸道损害,在养猪业中造成重大的经济损失。猪肺炎支原体免疫膜相关蛋白的表征可能在开发新的治疗方法中发挥作用。在这里,猪肺炎支原体的主要表面抗原蛋白之一P46的晶体结构呈现并显示了通过铰链连接的N和C末端α/β域。这项工作解决的结构包括P46的无配体开放形式(3.1Å分辨率)和P46的两个配体结合的结构,分别为麦芽糖(2.5Å分辨率)和木糖(3.5Å分辨率)开放和封闭构型。配体结合位点埋在铰链区域之间的缝隙中。P46的两个域可以相对旋转,从而提供开放或封闭的替代构象。与该结构信息一致,序列分析显示与ABC转运蛋白超家族的底物结合成员相似,其中P46作为功能性亚基面对细胞外。在具有木糖的结构中,P46还与高亲和力结合(K d = 29 nM)来自单克隆抗体的Fab片段,可表征P46中仅涉及C端结构域残基的结构表位。具有P46的复合物中的Fab结构在六个互补决定区(CDR)中相对于未结合的Fab仅显示出很小的构象重排(其结构在本研究中也以1.95Å的分辨率确定)。现在可用的结构信息应有助于更好地了解猪肺炎支原体对糖营养的摄入。该信息还将允许设计针对这种重要的猪病原体的新疫苗的产生方案和策略的设计。
更新日期:2020-05-01
中文翻译:

P46的结构,猪肺炎支原体的一种免疫优势表面蛋白:与单克隆抗体的相互作用。
猪肺炎支原体是一种原核病原体,定居在猪呼吸道纤毛上皮细胞中。被感染的动物遭受呼吸道损害,在养猪业中造成重大的经济损失。猪肺炎支原体免疫膜相关蛋白的表征可能在开发新的治疗方法中发挥作用。在这里,猪肺炎支原体的主要表面抗原蛋白之一P46的晶体结构呈现并显示了通过铰链连接的N和C末端α/β域。这项工作解决的结构包括P46的无配体开放形式(3.1Å分辨率)和P46的两个配体结合的结构,分别为麦芽糖(2.5Å分辨率)和木糖(3.5Å分辨率)开放和封闭构型。配体结合位点埋在铰链区域之间的缝隙中。P46的两个域可以相对旋转,从而提供开放或封闭的替代构象。与该结构信息一致,序列分析显示与ABC转运蛋白超家族的底物结合成员相似,其中P46作为功能性亚基面对细胞外。在具有木糖的结构中,P46还与高亲和力结合(K d = 29 nM)来自单克隆抗体的Fab片段,可表征P46中仅涉及C端结构域残基的结构表位。具有P46的复合物中的Fab结构在六个互补决定区(CDR)中相对于未结合的Fab仅显示出很小的构象重排(其结构在本研究中也以1.95Å的分辨率确定)。现在可用的结构信息应有助于更好地了解猪肺炎支原体对糖营养的摄入。该信息还将允许设计针对这种重要的猪病原体的新疫苗的产生方案和策略的设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号