当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal and solution structures of fragments of the human leucocyte common antigen-related protein.
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-05-01 , DOI: 10.1107/s2059798320003885 Joachim Vilstrup 1 , Amanda Simonsen 1 , Thea Birkefeldt 1 , Dorthe Strandbygård 1 , Jeppe Lyngsø 2 , Jan Skov Pedersen 2 , Søren Thirup 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-05-01 , DOI: 10.1107/s2059798320003885 Joachim Vilstrup 1 , Amanda Simonsen 1 , Thea Birkefeldt 1 , Dorthe Strandbygård 1 , Jeppe Lyngsø 2 , Jan Skov Pedersen 2 , Søren Thirup 1
Affiliation
|
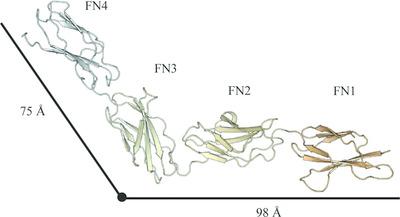
Leucocyte common antigen‐related protein (LAR) is a post‐synaptic type I transmembrane receptor protein that is important for neuronal functionality and is genetically coupled to neuronal disorders such as attention deficit hyperactivity disorder (ADHD). To understand the molecular function of LAR, structural and biochemical studies of protein fragments derived from the ectodomain of human LAR have been performed. The crystal structure of a fragment encompassing the first four FNIII domains (LARFN1–4) showed a characteristic L shape. SAXS data suggested limited flexibility within LARFN1–4, while rigid‐body refinement of the SAXS data using the X‐ray‐derived atomic model showed a smaller angle between the domains defining the L shape compared with the crystal structure. The capabilities of the individual LAR fragments to interact with heparin was examined using microscale thermophoresis and heparin‐affinity chromatography. The results showed that the three N‐terminal immunoglobulin domains (LARIg1–3) and the four C‐terminal FNIII domains (LARFN5–8) both bound heparin, while LARFN1–4 did not. The low‐molecular‐weight heparin drug Innohep induced a shift in hydrodynamic volume as assessed by size‐exclusion chromatography of LARIg1–3 and LARFN5–8, while the chemically defined pentameric heparin drug Arixtra did not. Together, the presented results suggest the presence of an additional heparin‐binding site in human LAR.
中文翻译:

人白细胞常见抗原相关蛋白片段的晶体和溶液结构。
白细胞共同抗原相关蛋白(LAR)是突触后的I型跨膜受体蛋白,对神经元功能很重要,并与神经元疾病(如注意力不足过动症(ADHD))遗传偶联。为了理解LAR的分子功能,已经进行了对源自人LAR的胞外域的蛋白质片段的结构和生化研究。包含前四个FNIII域(LAR FN1-4)的片段的晶体结构显示出特征L形。SAXS数据表明,LAR FN1-4中的灵活性有限,而使用X射线原子模型对SAXS数据进行刚体细化显示,与晶体结构相比,定义L形的畴之间的夹角较小。使用微尺度热泳和肝素亲和色谱法检查了各个LAR片段与肝素相互作用的能力。结果显示,三个N末端免疫球蛋白结构域(LAR Ig1-3)和四个C末端FNIII结构域(LAR FN5-8)都结合肝素,而LAR FN1-4没有结合肝素。低分子量肝素药物Innohep通过LAR Ig1–3和LAR FN5–8的体积排阻色谱法评估了流体动力学体积的变化,而化学定义的五聚体肝素药物Arixtra则没有。在一起,所提出的结果表明在人类LAR中存在一个额外的肝素结合位点。
更新日期:2020-05-01
中文翻译:

人白细胞常见抗原相关蛋白片段的晶体和溶液结构。
白细胞共同抗原相关蛋白(LAR)是突触后的I型跨膜受体蛋白,对神经元功能很重要,并与神经元疾病(如注意力不足过动症(ADHD))遗传偶联。为了理解LAR的分子功能,已经进行了对源自人LAR的胞外域的蛋白质片段的结构和生化研究。包含前四个FNIII域(LAR FN1-4)的片段的晶体结构显示出特征L形。SAXS数据表明,LAR FN1-4中的灵活性有限,而使用X射线原子模型对SAXS数据进行刚体细化显示,与晶体结构相比,定义L形的畴之间的夹角较小。使用微尺度热泳和肝素亲和色谱法检查了各个LAR片段与肝素相互作用的能力。结果显示,三个N末端免疫球蛋白结构域(LAR Ig1-3)和四个C末端FNIII结构域(LAR FN5-8)都结合肝素,而LAR FN1-4没有结合肝素。低分子量肝素药物Innohep通过LAR Ig1–3和LAR FN5–8的体积排阻色谱法评估了流体动力学体积的变化,而化学定义的五聚体肝素药物Arixtra则没有。在一起,所提出的结果表明在人类LAR中存在一个额外的肝素结合位点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号