当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cascading proton transfers are a hallmark of the catalytic mechanism of SAM‐dependent methyltransferases
FEBS Letters ( IF 3.0 ) Pub Date : 2020-05-16 , DOI: 10.1002/1873-3468.13799 Li Na Zhao 1 , Philipp Kaldis 1, 2
FEBS Letters ( IF 3.0 ) Pub Date : 2020-05-16 , DOI: 10.1002/1873-3468.13799 Li Na Zhao 1 , Philipp Kaldis 1, 2
Affiliation
|
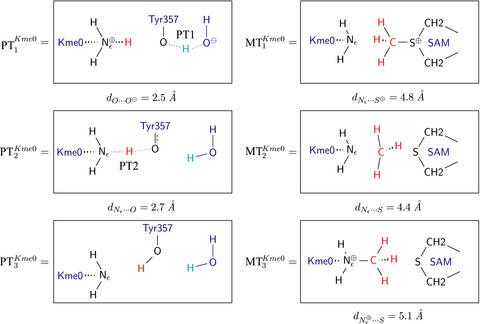
The S‐adenosyl methionine (SAM)‐dependent methyltransferases attach a methyl group to the deprotonated methyl lysine using SAM as a donor. An intriguing, yet unanswered, question is how the deprotonation takes place. PRDM9 with well‐defined enzyme activity is a good representative of the methyltransferase family to study the deprotonation and subsequently the methyl transfer. Our study has found that the pKa of Tyr357 is low enough to make it an ideal candidate for proton abstraction from the methyl lysine. The partially deprontonated Tyr357 is able to change its H‐bond pattern thus bridging two proton tunneling states and providing a cascading proton transfer. We have uncovered a new catalytic mechanism for the deprotonation of the methyl lysine in methyltransferases.
中文翻译:

级联质子转移是 SAM 依赖性甲基转移酶催化机制的标志
S-腺苷甲硫氨酸 (SAM) 依赖性甲基转移酶使用 SAM 作为供体将甲基连接到去质子化的甲基赖氨酸上。一个有趣但尚未解答的问题是去质子化是如何发生的。 PRDM9 具有明确的酶活性,是甲基转移酶家族的良好代表,可用于研究去质子化和随后的甲基转移。我们的研究发现 Tyr357 的 pKa 足够低,使其成为从甲基赖氨酸中提取质子的理想候选者。部分去质子化的 Tyr357 能够改变其氢键模式,从而桥接两个质子隧道状态并提供级联质子转移。我们发现了甲基转移酶中甲基赖氨酸去质子化的新催化机制。
更新日期:2020-05-16
中文翻译:

级联质子转移是 SAM 依赖性甲基转移酶催化机制的标志
S-腺苷甲硫氨酸 (SAM) 依赖性甲基转移酶使用 SAM 作为供体将甲基连接到去质子化的甲基赖氨酸上。一个有趣但尚未解答的问题是去质子化是如何发生的。 PRDM9 具有明确的酶活性,是甲基转移酶家族的良好代表,可用于研究去质子化和随后的甲基转移。我们的研究发现 Tyr357 的 pKa 足够低,使其成为从甲基赖氨酸中提取质子的理想候选者。部分去质子化的 Tyr357 能够改变其氢键模式,从而桥接两个质子隧道状态并提供级联质子转移。我们发现了甲基转移酶中甲基赖氨酸去质子化的新催化机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号