当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Spectroelectrochemical Investigation of Potential‐Dependent Changes in an Amphiphilic Imidazolium‐Based Ionic Liquid Film on the Au(111) Electrode Surface
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-05-05 , DOI: 10.1002/celc.202000385 Thorben Sieling 1 , Izabella Brand 1
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-05-05 , DOI: 10.1002/celc.202000385 Thorben Sieling 1 , Izabella Brand 1
Affiliation
|
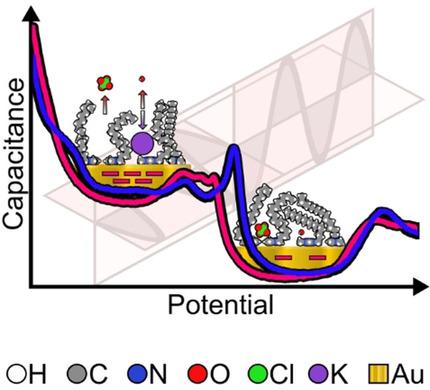
The stability of ionic liquids (ILs) at charged interfaces makes them very attractive in electrochemical applications. In this work, a Langmuir–Blodgett transfer is used to fabricate a monolayer of an amphiphilic 1‐methyl‐3‐octadecylimidazolium perchlorate IL on the Au(111) surface. The IL monolayer immersed in an aqueous electrolyte solution is very stable over a wide potential window. Polarization modulation infrared reflection absorption spectroscopy is used to monitor structural changes in the model of the electrical double layer of the IL in aqueous solution. The imidazolium cation is in direct contact with the Au(111) surface. The imidazolium ring adopts a rigid orientation, inclined toward the metal surface, in the monolayer on the Au(111) surface. The orientation of hydrocarbon chains responds to electric potentials. At low negative surface charge densities of the Au(111) electrode, the hydrocarbon chains in the amphiphilic cation have a large average tilt versus surface normal. A negative potential shift is accompanied by a reorientation of the hydrophobic hydrocarbon chains that adopt an up‐ward orientation, making the film permeable for counter‐ and/or co‐ions. This transition leads to the formation of an ordered, single‐component monolayer of the amphiphilic cation on the electrode surface.
中文翻译:

原位光谱电化学研究Au(111)电极表面两亲基于咪唑鎓的离子液体膜的电位依赖性变化
离子液体(ILs)在带电界面处的稳定性使其在电化学应用中非常有吸引力。在这项工作中,使用Langmuir-Blodgett转移在Au(111)表面上制造了两亲性的1两甲基3十八碳酰亚胺咪唑高氯酸盐IL单层。浸在电解质水溶液中的IL单层在很宽的电位窗口内非常稳定。偏振调制红外反射吸收光谱法用于监测水溶液中IL双电层模型的结构变化。咪唑鎓阳离子与Au(111)表面直接接触。咪唑环在Au(111)表面的单分子层中采用朝向金属表面倾斜的刚性取向。烃链的取向响应于电势。在Au(111)电极的负表面电荷密度低的情况下,两亲阳离子中的烃链相对于表面法线的平均倾斜度较大。负电位移动伴随着疏水烃链的重新取向,该疏水烃链采用向上的取向,使得该膜可渗透抗衡离子和/或共离子。这种转变导致在电极表面形成两亲阳离子的有序单组分单层。
更新日期:2020-05-05
中文翻译:

原位光谱电化学研究Au(111)电极表面两亲基于咪唑鎓的离子液体膜的电位依赖性变化
离子液体(ILs)在带电界面处的稳定性使其在电化学应用中非常有吸引力。在这项工作中,使用Langmuir-Blodgett转移在Au(111)表面上制造了两亲性的1两甲基3十八碳酰亚胺咪唑高氯酸盐IL单层。浸在电解质水溶液中的IL单层在很宽的电位窗口内非常稳定。偏振调制红外反射吸收光谱法用于监测水溶液中IL双电层模型的结构变化。咪唑鎓阳离子与Au(111)表面直接接触。咪唑环在Au(111)表面的单分子层中采用朝向金属表面倾斜的刚性取向。烃链的取向响应于电势。在Au(111)电极的负表面电荷密度低的情况下,两亲阳离子中的烃链相对于表面法线的平均倾斜度较大。负电位移动伴随着疏水烃链的重新取向,该疏水烃链采用向上的取向,使得该膜可渗透抗衡离子和/或共离子。这种转变导致在电极表面形成两亲阳离子的有序单组分单层。











































 京公网安备 11010802027423号
京公网安备 11010802027423号