当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction of tris(hydroxymethyl)phosphine and cinnamaldehyde in methanol
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-05-04 , DOI: 10.1002/jhet.3986 Dmitry V. Moiseev 1, 2 , Yulia B. Malysheva 2 , Aleksey V. Gushchin 2 , Brian R. James 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-05-04 , DOI: 10.1002/jhet.3986 Dmitry V. Moiseev 1, 2 , Yulia B. Malysheva 2 , Aleksey V. Gushchin 2 , Brian R. James 1
Affiliation
|
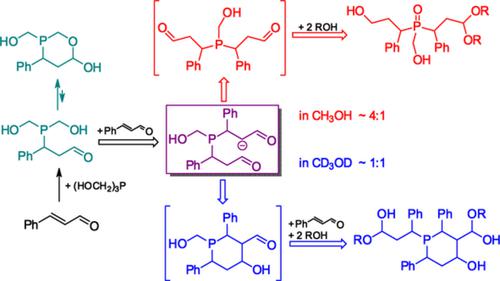
Reaction of tris(hydroxymethyl)phosphine with excess cinnamaldehyde in CH3OH or CD3OD, followed using NMR, proceeds via several phosphorus‐containing intermediates, multiple transformations of organic parts, and with the solvent H/D isotope effect on products. In both solvents, one CH2OH group of tris(hydroxymethyl)phosphine is readily replaced by the cinnamaldehyde moiety to give the primary product, a 1,3‐oxaphosphorinane derivative. Slower replacement of the second CH2OH group leads to a mixture of aliphatic and heterocyclic phosphine intermediates in a ratio of ~4:1 in CH3OH and ~1:1 in CD3OD; both intermediates contain alcohol and aldehyde groups and convert rapidly into intra‐ and intermolecular hemiacetals. The hemiacetals of the aliphatic phosphine rearrange further into an unsymmetrical trialkylphosphine oxide, whereas the hemiacetals of the heterocyclic phosphine react with the third mole of cinnamaldehyde to replace the third CH2OH group of tris(hydroxymethyl)phosphine. All intermediates and products are formed as mixtures of stereoisomers.
中文翻译:

三(羟甲基)膦与肉桂醛在甲醇中的反应
三(羟甲基)膦与过量的肉桂醛在CH 3 OH或CD 3 OD中的反应,然后使用NMR,通过几种含磷中间体,有机部分的多次转化以及溶剂H / D同位素对产物的影响而进行。在这两种溶剂中,三(羟甲基)膦的一个CH 2 OH基团都容易被肉桂醛部分取代,从而得到一级产物1,3-氧杂磷杂环丁烷衍生物。第二个CH 2 OH基团的缓慢取代导致脂肪族和杂环膦中间体的混合物在CH 3 OH中的比例为〜4:1,在CD 3中的比例为〜1:1OD; 两种中间体均含有醇和醛基,并迅速转化为分子内和分子间的半缩醛。脂族膦的半缩醛进一步重排成不对称的三烷基膦氧化物,而杂环膦的半缩醛与肉桂醛的第三摩尔反应以取代三(羟甲基)膦的第三CH 2 OH基团。所有中间体和产物均以立体异构体的混合物形式形成。
更新日期:2020-05-04
中文翻译:

三(羟甲基)膦与肉桂醛在甲醇中的反应
三(羟甲基)膦与过量的肉桂醛在CH 3 OH或CD 3 OD中的反应,然后使用NMR,通过几种含磷中间体,有机部分的多次转化以及溶剂H / D同位素对产物的影响而进行。在这两种溶剂中,三(羟甲基)膦的一个CH 2 OH基团都容易被肉桂醛部分取代,从而得到一级产物1,3-氧杂磷杂环丁烷衍生物。第二个CH 2 OH基团的缓慢取代导致脂肪族和杂环膦中间体的混合物在CH 3 OH中的比例为〜4:1,在CD 3中的比例为〜1:1OD; 两种中间体均含有醇和醛基,并迅速转化为分子内和分子间的半缩醛。脂族膦的半缩醛进一步重排成不对称的三烷基膦氧化物,而杂环膦的半缩醛与肉桂醛的第三摩尔反应以取代三(羟甲基)膦的第三CH 2 OH基团。所有中间体和产物均以立体异构体的混合物形式形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号