当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis and Biological Evaluation of Bengamide Analogues as ClpP Activators
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-05-05 , DOI: 10.1002/cjoc.202000133 Xue‐Qing Kong 1, 2 , Bing‐Yan Wei 1, 2 , Chen‐Xi Yu 1, 2 , Xiang‐Na Guan 1, 2 , Wei‐Ping Ma 1 , Gang Liu 1 , Cai‐Guang Yang 1 , Fa‐Jun Nan 1, 3
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-05-05 , DOI: 10.1002/cjoc.202000133 Xue‐Qing Kong 1, 2 , Bing‐Yan Wei 1, 2 , Chen‐Xi Yu 1, 2 , Xiang‐Na Guan 1, 2 , Wei‐Ping Ma 1 , Gang Liu 1 , Cai‐Guang Yang 1 , Fa‐Jun Nan 1, 3
Affiliation
|
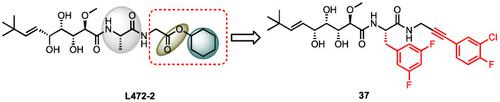
To combat multidrug‐resistant Gram‐positive bacteria, new antimicrobials particularly those with novel mechanism of action are badly needed. Different with conventional antibiotics which are typical inhibitors, small‐molecule activators of bacterial ClpP represent a new class of antibiotics. No ClpP activator has been developed for clinical trial. Herein, we conducted a screening on our library of bengamide‐like ring‐opened analogues and found that L472‐2 possesses a low minimum inhibitory concentration (MIC) against S.aureus and shows no activity for ClpP activation in vitro, but it displayed reduced antibacterial activity against S. aureus with clpP deletion. In order to obtain bengamide analogues that activate ClpP in vitro as well as possess antibacterial activity, we perform further structural modifications starting from L472‐2. Compound 37 remains the antimicrobial activity and activation of ClpP protein in vitro, which could be viewed as a new chemical scaffold for ClpP activators and worthy of further investigation.
中文翻译:

Bengamide类似物作为ClpP激活剂的设计,合成和生物学评估
为了对抗具有多重耐药性的革兰氏阳性细菌,迫切需要新的抗菌药物,尤其是具有新作用机制的抗菌药物。与典型的常规抗生素不同,细菌ClpP的小分子激活剂代表了一类新的抗生素。尚未开发用于临床试验的ClpP激活剂。在此,我们在我们的bengamide状的开环类似物文库进行了筛选,结果发现L472-2具有针对较低的最低抑制浓度(MIC)的金黄色葡萄球菌和示出了用于激活CLPP没有活性在体外,但它显示的缩小对抗菌活性金黄色葡萄球菌与CLPP删除。为了获得可在体外激活ClpP并具有抗菌活性的苯甲酰胺类似物,我们从L472-2开始进行进一步的结构修饰。化合物37在体外仍具有抗菌活性和对ClpP蛋白的激活作用,可作为ClpP激活剂的新型化学支架,值得进一步研究。
更新日期:2020-05-05
中文翻译:

Bengamide类似物作为ClpP激活剂的设计,合成和生物学评估
为了对抗具有多重耐药性的革兰氏阳性细菌,迫切需要新的抗菌药物,尤其是具有新作用机制的抗菌药物。与典型的常规抗生素不同,细菌ClpP的小分子激活剂代表了一类新的抗生素。尚未开发用于临床试验的ClpP激活剂。在此,我们在我们的bengamide状的开环类似物文库进行了筛选,结果发现L472-2具有针对较低的最低抑制浓度(MIC)的金黄色葡萄球菌和示出了用于激活CLPP没有活性在体外,但它显示的缩小对抗菌活性金黄色葡萄球菌与CLPP删除。为了获得可在体外激活ClpP并具有抗菌活性的苯甲酰胺类似物,我们从L472-2开始进行进一步的结构修饰。化合物37在体外仍具有抗菌活性和对ClpP蛋白的激活作用,可作为ClpP激活剂的新型化学支架,值得进一步研究。









































 京公网安备 11010802027423号
京公网安备 11010802027423号