当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Arenesulfonyl Fluoride Synthesis via Copper‐free Sandmeyer‐type Fluorosulfonylation of Arenediazonium Salts
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-05-05 , DOI: 10.1002/cjoc.202000175 Qiongzhen Lin 1 , Zhanhu Ma 2 , Changge Zheng 1 , Xiao‐Jun Hu 2 , Yong Guo 3 , Qing‐Yun Chen 3 , Chao Liu 2, 3
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-05-05 , DOI: 10.1002/cjoc.202000175 Qiongzhen Lin 1 , Zhanhu Ma 2 , Changge Zheng 1 , Xiao‐Jun Hu 2 , Yong Guo 3 , Qing‐Yun Chen 3 , Chao Liu 2, 3
Affiliation
|
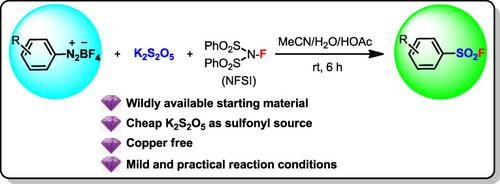
The limited availability of highly valuable arenesulfonyl fluorides seriously hinders their further application in many research fields including medicinal chemistry and chemical biological, organic synthesis, polymer preparation, etc. We report herein a mild and efficient copper‐free Sandmeyer‐type fluorosulfonylation reaction of various arenediazonium salts to prepare valuable arenesulfonyl fluorides using K2S2O5 as both a reductant and a practical sulfonyl source in combination with N‐fluorobenzenesulfonimide as an effective fluorine source. This methodology provides an attractive route to diverse important arenesulfonyl fluorides given the overall practicality and scope.
中文翻译:

Arenediazonium盐的无铜Sandmeyer型氟磺酰化反应合成芳烃磺酰氟
高价值的芳烃磺酰氟的有限供应严重阻碍了它们在许多研究领域的进一步应用,包括药物化学和化学生物学,有机合成,聚合物制备等。我们在此报告了一种温和而有效的各种铜氮杂唑鎓盐的无铜Sandmeyer型氟磺酰化反应,使用K 2 S 2 O 5作为还原剂和实用的磺酰基源结合N-氟苯磺酰亚胺作为有效的氟制备有价值的芳磺酰氟的方法。资源。考虑到总体实用性和范围,该方法学提供了一条吸引人的途径,可用于生产各种重要的芳烃磺酰氟。
更新日期:2020-05-05
中文翻译:

Arenediazonium盐的无铜Sandmeyer型氟磺酰化反应合成芳烃磺酰氟
高价值的芳烃磺酰氟的有限供应严重阻碍了它们在许多研究领域的进一步应用,包括药物化学和化学生物学,有机合成,聚合物制备等。我们在此报告了一种温和而有效的各种铜氮杂唑鎓盐的无铜Sandmeyer型氟磺酰化反应,使用K 2 S 2 O 5作为还原剂和实用的磺酰基源结合N-氟苯磺酰亚胺作为有效的氟制备有价值的芳磺酰氟的方法。资源。考虑到总体实用性和范围,该方法学提供了一条吸引人的途径,可用于生产各种重要的芳烃磺酰氟。











































 京公网安备 11010802027423号
京公网安备 11010802027423号