当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of new catalytic enantioselective formation of methylenelactam-based N,O-spirocyclic compounds via ring opening-asymmetric reclosure of hydroxylactams
Tetrahedron ( IF 2.1 ) Pub Date : 2020-05-05 , DOI: 10.1016/j.tet.2020.131252 Tetsuya Sengoku , Ayako Miyoshi , Tamaki Tsuda , Toshiyasu Inuzuka , Masami Sakamoto , Masaki Takahashi , Hidemi Yoda
中文翻译:

通过开环-不对称重合羟基内酰胺开发基于亚甲基内酰胺的N,O-螺环化合物的新催化对映选择性形成
更新日期:2020-05-05
Tetrahedron ( IF 2.1 ) Pub Date : 2020-05-05 , DOI: 10.1016/j.tet.2020.131252 Tetsuya Sengoku , Ayako Miyoshi , Tamaki Tsuda , Toshiyasu Inuzuka , Masami Sakamoto , Masaki Takahashi , Hidemi Yoda
|
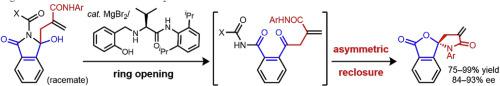
Catalytic enantioselective formation of methylenelactam-based N,O-spirocyclic compounds is developed. Hydroxylactams prepared from N-carbonyl phthalimides and β-amido functionalized allylboronates underwent ring opening-asymmetric reclosure in the presence of catalytic amounts of MgBr2 and a chiral aminophenol to afford the corresponding N,O-spirocyclic compounds in excellent yields and high enantioselectivities.
中文翻译:

通过开环-不对称重合羟基内酰胺开发基于亚甲基内酰胺的N,O-螺环化合物的新催化对映选择性形成
开发了基于亚甲基内酰胺的N,O-螺环化合物的催化对映选择性形成。由N-羰基邻苯二甲酰亚胺和β-氨基官能化的烯丙基硼酸酯制备的羟基内酰胺在催化量的MgBr 2和手性氨基苯酚存在下进行开环-不对称重合反应,从而以优异的收率和高对映选择性提供相应的N,O-螺环化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号