当前位置:
X-MOL 学术
›
BBA Bioenerg.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Charge polarization imposed by the binding site facilitates enzymatic redox reactions of quinone.
Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2020-05-05 , DOI: 10.1016/j.bbabio.2020.148216 Sebastian Pintscher 1 , Anna Wójcik-Augustyn 2 , Marcin Sarewicz 1 , Artur Osyczka 1
Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2020-05-05 , DOI: 10.1016/j.bbabio.2020.148216 Sebastian Pintscher 1 , Anna Wójcik-Augustyn 2 , Marcin Sarewicz 1 , Artur Osyczka 1
Affiliation
|
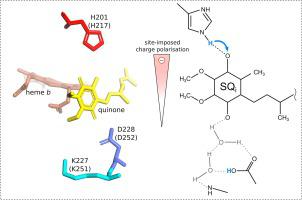
Quinone reduction site (Qi) of cytochrome bc1 represents one of the canonical sites used to explore the enzymatic redox reactions involving semiquinone (SQ) states. However, the mechanism by which Qi allows the completion of quinone reduction during the sequential transfers of two electrons from the adjacent heme bH and two protons to C1- and C4-carbonyl remains unclear. Here we established that the SQ coupled to an oxidized heme bH is a dominant intermediate of catalytic forward reaction and, contrary to the long-standing assumption, represents a significant population of SQ detected across pH 5-9. The pH dependence of its redox midpoint potential implicated proton exchange with histidine. Complementary quantum mechanical calculations revealed that the SQ anion formed after the first electron transfer undergoes charge and spin polarization imposed by the electrostatic field generated by histidine and the aspartate/lysine pair interacting with the C4- and C1-carbonyl, respectively. This favors a barrierless proton exchange between histidine and the C4-carbonyl, which continues until the second electron reaches the SQi. Inversion of charge polarization facilitates the uptake of the second proton by the C1-carbonyl. Based on these findings we developed a comprehensive scheme for electron and proton transfers at Qi featuring the equilibration between the anionic and neutral states of SQi as means for a leak-proof stabilization of the radical intermediate. The key catalytic role of the initial charge/spin polarization of the SQ anion at the active site, inherent to the proposed mechanism, may also be applicable to the other quinone oxidoreductases.
中文翻译:

由结合位点施加的电荷极化促进了醌的酶促氧化还原反应。
细胞色素bc1的醌还原位点(Qi)代表用于研究涉及半醌(SQ)状态的酶促氧化还原反应的规范位点之一。然而,在两个电子从相邻的血红素bH和两个质子依次转移到C1-和C4-羰基的过程中,Qi可以完成醌还原的机理尚不清楚。在这里,我们确定了与氧化血红素bH偶联的SQ是催化正向反应的主要中间体,与长期以来的假设相反,它代表了在pH 5-9范围内检测到的大量SQ。其氧化还原中点电势的pH依赖性涉及与组氨酸的质子交换。互补的量子力学计算表明,第一次电子转移后形成的SQ阴离子经历电荷和自旋极化,这是由组氨酸和天冬氨酸/赖氨酸对分别与C4-和C1-羰基相互作用产生的静电场施加的。这有利于组氨酸和C4-羰基之间的无障碍质子交换,该交换一直持续到第二个电子到达SQi为止。电荷极化的反转促进了C1-羰基对第二个质子的吸收。基于这些发现,我们开发了一种用于Qi处电子和质子转移的综合方案,其特征在于SQi的阴离子和中性状态之间的平衡,是防止自由基中间体稳定泄漏的手段。
更新日期:2020-05-05
中文翻译:

由结合位点施加的电荷极化促进了醌的酶促氧化还原反应。
细胞色素bc1的醌还原位点(Qi)代表用于研究涉及半醌(SQ)状态的酶促氧化还原反应的规范位点之一。然而,在两个电子从相邻的血红素bH和两个质子依次转移到C1-和C4-羰基的过程中,Qi可以完成醌还原的机理尚不清楚。在这里,我们确定了与氧化血红素bH偶联的SQ是催化正向反应的主要中间体,与长期以来的假设相反,它代表了在pH 5-9范围内检测到的大量SQ。其氧化还原中点电势的pH依赖性涉及与组氨酸的质子交换。互补的量子力学计算表明,第一次电子转移后形成的SQ阴离子经历电荷和自旋极化,这是由组氨酸和天冬氨酸/赖氨酸对分别与C4-和C1-羰基相互作用产生的静电场施加的。这有利于组氨酸和C4-羰基之间的无障碍质子交换,该交换一直持续到第二个电子到达SQi为止。电荷极化的反转促进了C1-羰基对第二个质子的吸收。基于这些发现,我们开发了一种用于Qi处电子和质子转移的综合方案,其特征在于SQi的阴离子和中性状态之间的平衡,是防止自由基中间体稳定泄漏的手段。











































 京公网安备 11010802027423号
京公网安备 11010802027423号