当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Copper-Catalyzed Remote C(sp3)-H Alkynylation of Linear Primary Sulfonamides.
Organic Letters ( IF 4.9 ) Pub Date : 2020-05-05 , DOI: 10.1021/acs.orglett.0c01325 Cheng-Yu Wang 1 , Zi-Yang Qin 1 , Yu-Ling Huang 1 , Yi-Ming Hou 1 , Ruo-Xing Jin 1 , Chao Li 1 , Xi-Sheng Wang 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-05-05 , DOI: 10.1021/acs.orglett.0c01325 Cheng-Yu Wang 1 , Zi-Yang Qin 1 , Yu-Ling Huang 1 , Yi-Ming Hou 1 , Ruo-Xing Jin 1 , Chao Li 1 , Xi-Sheng Wang 1
Affiliation
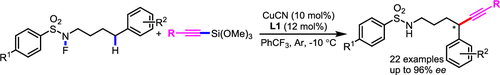
|
The highly efficient copper-catalyzed enantioselective alkynylation of the remote C(sp3)-H bond on linear primary sulfonamides is presented here using a radical relay strategy. The chiral box-copper complex, which is used to recapture the in-situ-generated alkyl radical via a 1,5-HAT strategy, is the key to success, affording the chiral alkynes after a following reductive elimination. A general substrate scope, mild conditions, and excellent regio- and enantioselective control are demonstrated in this method.
中文翻译:

线性伯磺酰胺的对映选择性铜催化的远程C(sp3)-H烷基化。
此处使用自由基中继策略介绍了高效的铜催化线性伯磺酰胺上远程C(sp3)-H键的铜对映体选择性炔基化反应。手性盒铜配合物是成功的关键,该手性盒铜配合物可通过1,5-HAT策略重新捕获原位生成的烷基,在还原反应消除后得到手性炔烃。该方法证明了一般的底物范围,温和的条件以及出色的区域和对映选择性控制。
更新日期:2020-05-05
中文翻译:

线性伯磺酰胺的对映选择性铜催化的远程C(sp3)-H烷基化。
此处使用自由基中继策略介绍了高效的铜催化线性伯磺酰胺上远程C(sp3)-H键的铜对映体选择性炔基化反应。手性盒铜配合物是成功的关键,该手性盒铜配合物可通过1,5-HAT策略重新捕获原位生成的烷基,在还原反应消除后得到手性炔烃。该方法证明了一般的底物范围,温和的条件以及出色的区域和对映选择性控制。









































 京公网安备 11010802027423号
京公网安备 11010802027423号