当前位置:
X-MOL 学术
›
Chem. Ing. Tech.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Calculating the Reaction Order and Activation Energy for the Hydrothermal Carbonization of Fructose
Chemie Ingenieur Technik ( IF 1.5 ) Pub Date : 2020-05-04 , DOI: 10.1002/cite.201900093 Dennis Jung 1 , Paul Körner 1 , Andrea Kruse 1
Chemie Ingenieur Technik ( IF 1.5 ) Pub Date : 2020-05-04 , DOI: 10.1002/cite.201900093 Dennis Jung 1 , Paul Körner 1 , Andrea Kruse 1
Affiliation
|
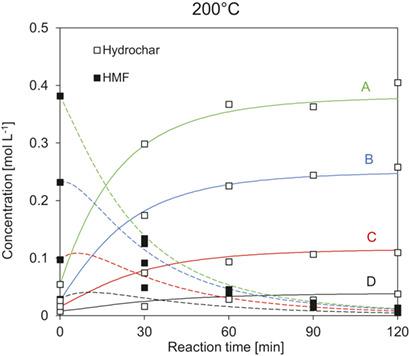
The development of accurate kinetic models for hydrothermal carbonization (HTC) faces major difficulties. This is related to the fact that the formation of the solid hydrochar can be regarded as a formation of a second phase from a homogenous solution. The reaction mechanism is not fully understood. From the current state of art, it is obvious that the reaction mechanism is some sort of polymerization reaction, thus, having reaction order higher than one. In order to gain more knowledge about the HTC reaction, fructose is used as a model substance, as it can be regarded as a key intermediate during the hydrothermal processing of carbohydrates. The results indicate that the reaction order is a highly sensitive parameter varying with temperature, reaction time and initial concentration. It can be concluded that the model itself is a strong simplification of the real reaction mechanism, thus more research is necessary to complete the actual knowledge.
中文翻译:

果糖水热碳化反应阶数和活化能的计算
开发精确的热液碳化动力学模型面临重大困难。这与以下事实有关:可以将固体水炭的形成视为由均质溶液形成的第二相。反应机理尚不完全清楚。从目前的技术水平来看,显然反应机理是某种聚合反应,因此,其反应级高于一个。为了获得更多有关HTC反应的知识,果糖被用作模型物质,因为它可以被视为碳水化合物的水热加工过程中的关键中间体。结果表明,反应顺序是随温度,反应时间和初始浓度而变化的高度敏感的参数。
更新日期:2020-05-04
中文翻译:

果糖水热碳化反应阶数和活化能的计算
开发精确的热液碳化动力学模型面临重大困难。这与以下事实有关:可以将固体水炭的形成视为由均质溶液形成的第二相。反应机理尚不完全清楚。从目前的技术水平来看,显然反应机理是某种聚合反应,因此,其反应级高于一个。为了获得更多有关HTC反应的知识,果糖被用作模型物质,因为它可以被视为碳水化合物的水热加工过程中的关键中间体。结果表明,反应顺序是随温度,反应时间和初始浓度而变化的高度敏感的参数。











































 京公网安备 11010802027423号
京公网安备 11010802027423号