当前位置:
X-MOL 学术
›
Eur. J. Lipid Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Docking Reveals the Binding Modes of Anticancer Alkylphospholipids and Lysophosphatidylcholine within the Catalytic Domain of Cytidine Triphosphate: Phosphocholine Cytidyltransferase
European Journal of Lipid Science and Technology ( IF 1.8 ) Pub Date : 2020-06-11 , DOI: 10.1002/ejlt.201900422 Xisto Antonio de Oliveira Neto 1 , Anna Carolina Schneider Alves 1 , Reinaldo Antonio Dias Junior 2 , Ricardo Pereira Rodrigues 3 , Marcelo Lancellotti 1 , Wanda Pereira Almeida 1 , Daniel Fábio Kawano 1, 2
European Journal of Lipid Science and Technology ( IF 1.8 ) Pub Date : 2020-06-11 , DOI: 10.1002/ejlt.201900422 Xisto Antonio de Oliveira Neto 1 , Anna Carolina Schneider Alves 1 , Reinaldo Antonio Dias Junior 2 , Ricardo Pereira Rodrigues 3 , Marcelo Lancellotti 1 , Wanda Pereira Almeida 1 , Daniel Fábio Kawano 1, 2
Affiliation
|
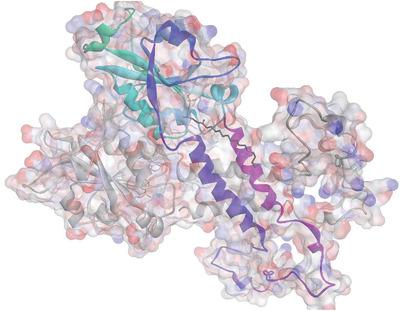
Alkylphospholipids are synthetic analogues of endogenous phosphatidylcholines with a remarkable ability: induce the selective apoptosis of exponentially growing tumor cells. One hypothesis concerning their mechanism of action is the inhibition of cytidine triphosphate:phosphocholine cytidyltransferase (CCT), which would significantly suppress the phosphatidylcholine biosynthesis to trigger apoptosis. Herein, homology modeling, docking simulations, and the analyses of molecular interaction fields are used to suggest the most probable binding modes of four alkylphospholipids (edelfosine, erucylphosphocholine, perifosine, and miltefosine) and lysophosphatidylcholine at the catalytic domain of human CCT. All compounds display bind modes in agreement with the corresponding groups found in the CCT substrate, phosphocholine, while their binding strengths are increased because of the interaction of the alkyl chains with hydrophobic residues from the M domain of the protein. Analyses of the geometry of the CCT binding‐site also suggest that small groups, such as benzyl/2‐phenylethyl ethers or equivalent heterocycles, could replace the O‐methyl group in edelfosine to yield even better inhibitors. It is believed this study can guide the development of new alkylphospholipids with an improved profile for the inhibition of phosphatidylcholine biosynthesis, a critical component for cell cycle progression that can be explored in cancer chemotherapy.
中文翻译:

分子对接揭示了三磷酸胞苷催化域内的抗癌烷基磷脂和溶血磷脂酰胆碱的结合模式:磷酸胆碱胞苷转移酶
烷基磷脂是具有显着能力的内源性磷脂酰胆碱的合成类似物:诱导呈指数增长的肿瘤细胞的选择性凋亡。关于它们的作用机理的一种假设是抑制胞苷三磷酸:磷酸胆碱的胞苷基转移酶(CCT),其将显着抑制磷脂酰胆碱的生物合成以触发细胞凋亡。在这里,同源性建模,对接模拟和分子相互作用领域的分析被用来表明在人类CCT催化域的四个烷基磷脂(edelfosine,芥子酰磷酸胆碱,periposine和miltefosine)和溶血磷脂酰胆碱的最可能结合模式。所有化合物的结合方式均与CCT底物,磷酸胆碱,由于烷基链与蛋白质M结构域中的疏水残基相互作用,它们的结合强度提高了。对CCT结合位点的几何分析也表明,小基团(例如苄基-2-苯乙基醚或等效的杂环)可以取代依德福星中的O-甲基,从而产生更好的抑制剂。相信这项研究可以指导开发具有改善的抑制磷脂酰胆碱生物合成特性的新的烷基磷脂,磷脂酰胆碱的生物合成是细胞周期发展的关键组成部分,可以在癌症化疗中进行探索。例如苄基/ 2-苯基乙基醚或等效的杂环,可以取代依德福星中的O-甲基,从而产生更好的抑制剂。相信这项研究可以指导开发具有改善的抑制磷脂酰胆碱生物合成特性的新的烷基磷脂,磷脂酰胆碱的生物合成是细胞周期发展的关键组成部分,可以在癌症化疗中进行探索。例如苄基/ 2-苯基乙基醚或等效的杂环,可以取代依德福星中的O-甲基,从而产生更好的抑制剂。相信这项研究可以指导开发具有改善的抑制磷脂酰胆碱生物合成特性的新的烷基磷脂,磷脂酰胆碱的生物合成是细胞周期发展的关键组成部分,可以在癌症化疗中进行探索。
更新日期:2020-06-11
中文翻译:

分子对接揭示了三磷酸胞苷催化域内的抗癌烷基磷脂和溶血磷脂酰胆碱的结合模式:磷酸胆碱胞苷转移酶
烷基磷脂是具有显着能力的内源性磷脂酰胆碱的合成类似物:诱导呈指数增长的肿瘤细胞的选择性凋亡。关于它们的作用机理的一种假设是抑制胞苷三磷酸:磷酸胆碱的胞苷基转移酶(CCT),其将显着抑制磷脂酰胆碱的生物合成以触发细胞凋亡。在这里,同源性建模,对接模拟和分子相互作用领域的分析被用来表明在人类CCT催化域的四个烷基磷脂(edelfosine,芥子酰磷酸胆碱,periposine和miltefosine)和溶血磷脂酰胆碱的最可能结合模式。所有化合物的结合方式均与CCT底物,磷酸胆碱,由于烷基链与蛋白质M结构域中的疏水残基相互作用,它们的结合强度提高了。对CCT结合位点的几何分析也表明,小基团(例如苄基-2-苯乙基醚或等效的杂环)可以取代依德福星中的O-甲基,从而产生更好的抑制剂。相信这项研究可以指导开发具有改善的抑制磷脂酰胆碱生物合成特性的新的烷基磷脂,磷脂酰胆碱的生物合成是细胞周期发展的关键组成部分,可以在癌症化疗中进行探索。例如苄基/ 2-苯基乙基醚或等效的杂环,可以取代依德福星中的O-甲基,从而产生更好的抑制剂。相信这项研究可以指导开发具有改善的抑制磷脂酰胆碱生物合成特性的新的烷基磷脂,磷脂酰胆碱的生物合成是细胞周期发展的关键组成部分,可以在癌症化疗中进行探索。例如苄基/ 2-苯基乙基醚或等效的杂环,可以取代依德福星中的O-甲基,从而产生更好的抑制剂。相信这项研究可以指导开发具有改善的抑制磷脂酰胆碱生物合成特性的新的烷基磷脂,磷脂酰胆碱的生物合成是细胞周期发展的关键组成部分,可以在癌症化疗中进行探索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号