Chem ( IF 23.5 ) Pub Date : 2020-05-04 , DOI: 10.1016/j.chempr.2020.04.006 Gang-Wei Wang , Matthew Wheatley , Marco Simonetti , Diego M. Cannas , Igor Larrosa
|
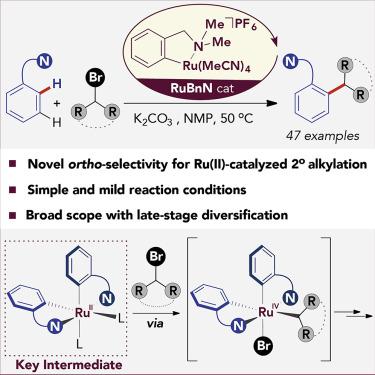
Although Ru-catalyzed meta-selective sp2 C–H alkylation with secondary alkyl halides is well established, ortho selectivity has never been achieved. We demonstrate that the use of a cyclometalated Ru-complex, RuBnN, as the catalyst results in a complete switch of the inherent meta-selectivity to ortho selectivity in the Ru-catalyzed sp2 C–H alkylation reaction with unactivated secondary alkyl halides. The high catalytic activity of RuBnN allows mild reaction conditions that result in a transformation of broad scope and versatility. Preliminary mechanistic studies suggest that a bis-cycloruthenated species is the key intermediate undergoing oxidative addition with the alkyl bromides, thus avoiding the more common SET pathway associated with meta-selectivity.
中文翻译:

环金属化钌催化剂可与仲烷基溴化物进行邻位选择性C–H烷基化
尽管已经很好地确定了Ru催化的具有仲烷基卤化物的间选择性sp 2 C–H烷基化反应,但邻位选择性从未实现。我们证明,使用的环金属钌络合物,RuBnN,如在固有的一个完整的开关催化剂结果元-选择性到邻位选择性Ru-催化SP 2C–H烷基化反应与未活化的仲烷基卤化物。RuBnN的高催化活性允许温和的反应条件,导致宽范围和多功能性的转变。初步的机理研究表明,双环钌化物种是与烷基溴发生氧化加成反应的关键中间体,因此避免了与间位选择性相关的更常见的SET途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号