当前位置:
X-MOL 学术
›
Chin. J. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ag2−O with highly exposed {111} crystal facets for efficient electrochemical oxygen evolution: Activity and mechanism
Chinese Journal of Catalysis ( IF 16.5 ) Pub Date : 2020-11-01 , DOI: 10.1016/s1872-2067(20)63574-4 Xiao-Feng Zhang , Jian-Sheng Li , Wan-Sheng You , Zai-Ming Zhu
Chinese Journal of Catalysis ( IF 16.5 ) Pub Date : 2020-11-01 , DOI: 10.1016/s1872-2067(20)63574-4 Xiao-Feng Zhang , Jian-Sheng Li , Wan-Sheng You , Zai-Ming Zhu
|
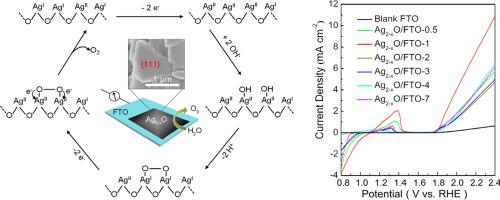
Abstract A series of Ag2–xO/FTO-i electrodes (where i denotes the current density during the electrodeposition, and i = 0.5, 1, 2, 3, 4, or 7) was fabricated in 0.1 M K2B4O7 electrolyte containing Ag+ ions by galvanostatic electrocrystallization. The electrode composition and morphology were characterized using X-ray powder diffraction, scanning electron microscopy, and X-ray photoelectron spectroscopy. The results reveal that the electrode films consist of Ag2O, but some of the Ag+ ions on the {111} crystal facets are oxidized into Ag2+ ions. Furthermore, the Ag2–xO/FTO-1 electrode shows a triangular slice shape of a parallel matrix with a larger exposed area of {111} crystal facets than other Ag2–xO/FTO-i (i = 0.5, 2, 3, 4, or 7) electrodes. Electrocatalytic experiments prove that the Ag2−xO/FTO-1 electrode produces the highest oxidative current density, has an overpotential of 417 mV at 10 mA cm−2, and has a Tafel slope of 47 mV dec−1 in 0.1 M K2B4O7. Electrochemical impedance spectra indicate that Ag2−xO/FTO-1 electrodes have the best ability for charge transfer. In addition, in the I-t test over 10 h, the current density decreased 4%. Fortunately, both O–O and Ag2+ species were detected after electrocatalysis and a possible mechanism for the oxygen evolution reaction is proposed in which the formation of Ag2+ and O–O species on {111} facets plays a critical role.
中文翻译:

具有高度暴露的 {111} 晶面的 Ag2-O 用于高效电化学析氧:活性和机制
摘要 在含有 Ag+ 离子的 0.1 M K2B4O7 电解液中制备了一系列 Ag2-xO/FTO-i 电极(其中 i 表示电沉积过程中的电流密度,i = 0.5、1、2、3、4 或 7)恒电流电结晶。使用 X 射线粉末衍射、扫描电子显微镜和 X 射线光电子能谱表征电极组成和形貌。结果表明,电极膜由 Ag2O 组成,但 {111} 晶面上的一些 Ag+ 离子被氧化成 Ag2+ 离子。此外,Ag2-xO/FTO-1 电极显示出平行矩阵的三角形切片形状,{111} 晶面的暴露面积比其他 Ag2-xO/FTO-i (i = 0.5, 2, 3, 4 , 或 7) 电极。电催化实验证明,Ag2-xO/FTO-1 电极产生最高的氧化电流密度,在 10 mA cm-2 时具有 417 mV 的过电位,在 0.1 M K2B4O7 中具有 47 mV dec-1 的 Tafel 斜率。电化学阻抗谱表明 Ag2-xO/FTO-1 电极具有最好的电荷转移能力。此外,在超过 10 小时的 It 测试中,电流密度下降了 4%。幸运的是,在电催化后检测到 O-O 和 Ag2+ 物种,并提出了析氧反应的可能机制,其中 {111} 面上的 Ag2+ 和 O-O 物种的形成起着关键作用。此外,在超过 10 小时的 It 测试中,电流密度下降了 4%。幸运的是,在电催化后检测到 O-O 和 Ag2+ 物种,并提出了析氧反应的可能机制,其中 {111} 面上的 Ag2+ 和 O-O 物种的形成起着关键作用。此外,在超过 10 小时的 It 测试中,电流密度下降了 4%。幸运的是,在电催化后检测到 O-O 和 Ag2+ 物种,并提出了析氧反应的可能机制,其中 {111} 面上的 Ag2+ 和 O-O 物种的形成起着关键作用。
更新日期:2020-11-01
中文翻译:

具有高度暴露的 {111} 晶面的 Ag2-O 用于高效电化学析氧:活性和机制
摘要 在含有 Ag+ 离子的 0.1 M K2B4O7 电解液中制备了一系列 Ag2-xO/FTO-i 电极(其中 i 表示电沉积过程中的电流密度,i = 0.5、1、2、3、4 或 7)恒电流电结晶。使用 X 射线粉末衍射、扫描电子显微镜和 X 射线光电子能谱表征电极组成和形貌。结果表明,电极膜由 Ag2O 组成,但 {111} 晶面上的一些 Ag+ 离子被氧化成 Ag2+ 离子。此外,Ag2-xO/FTO-1 电极显示出平行矩阵的三角形切片形状,{111} 晶面的暴露面积比其他 Ag2-xO/FTO-i (i = 0.5, 2, 3, 4 , 或 7) 电极。电催化实验证明,Ag2-xO/FTO-1 电极产生最高的氧化电流密度,在 10 mA cm-2 时具有 417 mV 的过电位,在 0.1 M K2B4O7 中具有 47 mV dec-1 的 Tafel 斜率。电化学阻抗谱表明 Ag2-xO/FTO-1 电极具有最好的电荷转移能力。此外,在超过 10 小时的 It 测试中,电流密度下降了 4%。幸运的是,在电催化后检测到 O-O 和 Ag2+ 物种,并提出了析氧反应的可能机制,其中 {111} 面上的 Ag2+ 和 O-O 物种的形成起着关键作用。此外,在超过 10 小时的 It 测试中,电流密度下降了 4%。幸运的是,在电催化后检测到 O-O 和 Ag2+ 物种,并提出了析氧反应的可能机制,其中 {111} 面上的 Ag2+ 和 O-O 物种的形成起着关键作用。此外,在超过 10 小时的 It 测试中,电流密度下降了 4%。幸运的是,在电催化后检测到 O-O 和 Ag2+ 物种,并提出了析氧反应的可能机制,其中 {111} 面上的 Ag2+ 和 O-O 物种的形成起着关键作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号