当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploiting cation aggregation in new magnesium amidohaloaluminate electrolytes for magnesium batteries
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2020-05-01 , DOI: 10.1039/c9qi01606f Etienne V. Brouillet 1, 2, 3, 4, 5 , Marco Amores 6, 7, 8, 9, 10 , Serena A. Corr 5, 11, 12, 13, 14 , Stuart D. Robertson 1, 2, 3, 4, 5
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2020-05-01 , DOI: 10.1039/c9qi01606f Etienne V. Brouillet 1, 2, 3, 4, 5 , Marco Amores 6, 7, 8, 9, 10 , Serena A. Corr 5, 11, 12, 13, 14 , Stuart D. Robertson 1, 2, 3, 4, 5
Affiliation
|
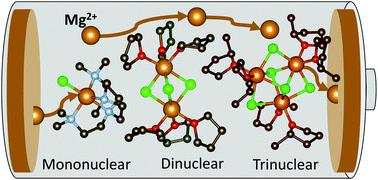
Mg batteries present an attractive and sustainable alternative to Li-ion batteries, wherein magnesium metal as an anode displays a superior theoretical volumetric energy density of 3833 A h L−1versus 2062 A h L−1 for lithium. An outstanding crucial bottleneck in realising their more widespread uptake is the development of suitable electrolytes, where electrode passivation, a limited electrochemical window, conditioning requirements, low ion mobility and low coulombic efficiencies all contribute to current limitations in Mg batteries. In an area thus far dominated by the thermodynamically stable [Mg2Cl3]+ dinuclear cation, we present here a novel family of magnesium amidohaloaluminate electrolytes [(Dipp)(SiMe3)2NAlCl3]− [MgxCl2x−1]+ where the magnesium chloride cation aggregation has been tailored (x = 1, 2, 3) by substitution of the coordinating ligand to the Mg2+ centre, and show how directly altering this cation affects battery performance (Dipp = 2,6-diisopropylphenyl, Me = methyl). The electrochemical activity of these new electrolytes has been evaluated by cyclic voltammetry, galvanostatic cycling and impedance spectroscopy in Mg–metal symmetrical cells as well as in battery cells with the Mo6S8 Chevrel phase cathode material against magnesium metal. The mononuclear and dinuclear magnesium amidohaloaluminate electrolytes facilitate reversible Mg plating and stripping from the Mg–metal anode with excellent stability, withstanding over 70 hours of continuous cycling. We demonstrate the compatibility of these novel electrolytes with the Mo6S8 Chevrel intercalation cathode material, allowing cycling of a Mg–metal cell up to 100 cycles with coulombic efficiencies above 95%.
中文翻译:

在用于镁电池的新型氨基卤铝铝酸镁电解质中利用阳离子聚集
镁电池是锂离子电池的一种有吸引力且可持续的替代品,其中,作为阳极的镁金属显示出的锂理论容量能量密度为3833 A h L -1,而锂为2062 A h L -1。实现其更广泛吸收的一个重要的关键瓶颈是合适的电解质的开发,其中电极钝化,有限的电化学窗口,调节要求,低离子迁移率和低库仑效率都造成了镁电池的电流限制。在迄今为止热力学稳定的[Mg 2 Cl 3 ] +双核阳离子,我们在这里介绍一种新型的氨基卤代铝酸镁电解质[(Dipp)(SiMe 3)2 NAlCl 3 ] - [Mg x Cl 2 x -1 ] +,其中已定制了氯化镁阳离子的聚集体(x = 1, 2,3)通过将配位体取代为Mg 2+中心,并显示直接改变这种阳离子如何影响电池性能(Dipp = 2,6-二异丙基苯基,Me =甲基)。这些新电解质的电化学活性已通过循环伏安法,恒电流循环和阻抗光谱法在Mg-金属对称电池以及含Mo 6 S 8 Chevrel相阴极材料对镁金属的电池中进行了评估。单核和双核氨基卤铝铝酸盐电解质可逆地进行Mg电镀和从Mg-金属阳极上剥离,具有出色的稳定性,可承受70个小时以上的连续循环。我们证明了这些新型电解质与Mo 6 S 8的相容性 Chevrel嵌入阴极材料,允许Mg-金属电池循环多达100个循环,库仑效率超过95%。
更新日期:2020-05-01
中文翻译:

在用于镁电池的新型氨基卤铝铝酸镁电解质中利用阳离子聚集
镁电池是锂离子电池的一种有吸引力且可持续的替代品,其中,作为阳极的镁金属显示出的锂理论容量能量密度为3833 A h L -1,而锂为2062 A h L -1。实现其更广泛吸收的一个重要的关键瓶颈是合适的电解质的开发,其中电极钝化,有限的电化学窗口,调节要求,低离子迁移率和低库仑效率都造成了镁电池的电流限制。在迄今为止热力学稳定的[Mg 2 Cl 3 ] +双核阳离子,我们在这里介绍一种新型的氨基卤代铝酸镁电解质[(Dipp)(SiMe 3)2 NAlCl 3 ] - [Mg x Cl 2 x -1 ] +,其中已定制了氯化镁阳离子的聚集体(x = 1, 2,3)通过将配位体取代为Mg 2+中心,并显示直接改变这种阳离子如何影响电池性能(Dipp = 2,6-二异丙基苯基,Me =甲基)。这些新电解质的电化学活性已通过循环伏安法,恒电流循环和阻抗光谱法在Mg-金属对称电池以及含Mo 6 S 8 Chevrel相阴极材料对镁金属的电池中进行了评估。单核和双核氨基卤铝铝酸盐电解质可逆地进行Mg电镀和从Mg-金属阳极上剥离,具有出色的稳定性,可承受70个小时以上的连续循环。我们证明了这些新型电解质与Mo 6 S 8的相容性 Chevrel嵌入阴极材料,允许Mg-金属电池循环多达100个循环,库仑效率超过95%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号