Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jmb.2020.04.025 Volkan Ergin 1 , Sika Zheng 1
|
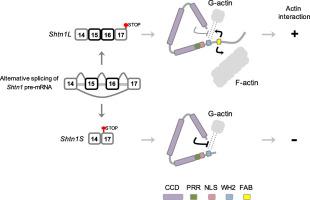
The actin cytoskeleton plays a pivotal role in cell development, morphogenesis, and other cellular functions. Precise control of actin dynamics requires actin-binding proteins. Here, we characterize multifarious regulation of SHTN1 (shootin1) and show that, unlike known actin-binding proteins, SHTN1’s actin binding activity is intrinsically inhibited by a putative coiled-coil domain (CCD) and the autoinhibition is overcome by alternative splicing regulation. We found SHTN1 contains a noncanonical WH2 domain and an upstream proline-rich region (PRR) that by themselves are sufficient for actin interaction. Alternative splicing of Shtn1 at the C terminus and downstream of the WH2-PRR domain produces a long (SHTN1L or shootin1b) and a short (SHTN1S or shootin1a) isoform, which both contain the described PRR and WH2 domains. However, SHTN1S does not interact with actin due to inhibition mediated by an N-terminal CCD. A SHTN1L-specific C-terminal motif counters the intramolecular inhibition and allows SHNT1L to bind actin. A nuclear localization signal is embedded between PRR and WH2 and is subject to similar autoinhibition. SHTN1 would be the first WH2-containing molecule that adopts CCD-dependent autoinhibition and alternative splicing-dependent actin interaction.
中文翻译:

推定的螺旋线圈域依赖性自动抑制和选择性剪接确定SHTN1的肌动蛋白结合活性。
肌动蛋白的细胞骨架在细胞发育,形态发生和其他细胞功能中起关键作用。肌动蛋白动力学的精确控制需要肌动蛋白结合蛋白。在这里,我们表征SHTN1(shootin1)的多种调控,并表明,与已知的肌动蛋白结合蛋白不同,SHTN1的肌动蛋白结合活性被推定的卷曲螺旋结构域(CCD)固有地抑制,并且通过选择性剪接调控克服了自抑制作用。我们发现SHTN1包含一个非规范的WH2域和一个上游的富含脯氨酸的区域(PRR),它们本身就足以进行肌动蛋白相互作用。Shtn1的替代剪接在WH2-PRR域的C末端和下游产生一个长的(SHTN1L或Shootin1b)和一个短的(SHTN1S或Shootin1a)同工型,它们均包含所述的PRR和WH2域。但是,由于N端CCD介导的抑制作用,SHTN1S不与肌动蛋白相互作用。SHTN1L特异的C端基元抵消了分子内的抑制作用,并使SHNT1L结合肌动蛋白。核定位信号嵌入在PRR和WH2之间,并受到类似的自抑制作用。SHTN1将是第一个通过CCD依赖自动抑制和替代剪接依赖肌动蛋白相互作用的WH2分子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号