当前位置:
X-MOL 学术
›
J. Mol. Cell. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Extracellular vesicular MicroRNA-27a* contributes to cardiac hypertrophy in chronic heart failure.
Journal of Molecular and Cellular Cardiology ( IF 4.9 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.yjmcc.2020.04.032 Changhai Tian 1 , Guoku Hu 2 , Lie Gao 1 , Bryan T Hackfort 1 , Irving H Zucker 1
Journal of Molecular and Cellular Cardiology ( IF 4.9 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.yjmcc.2020.04.032 Changhai Tian 1 , Guoku Hu 2 , Lie Gao 1 , Bryan T Hackfort 1 , Irving H Zucker 1
Affiliation
|
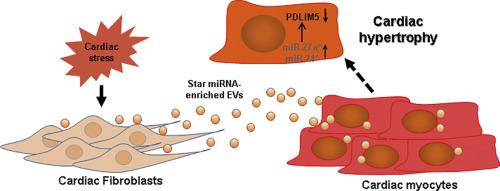
Under stress, the heart undergoes extensive remodeling resulting in cardiac fibrosis and hypertrophy, ultimately contributing to chronic heart failure (CHF). Alterations in microRNA levels are associated with dysfunctional gene expression profiles involved in the pathogenesis of heart failure. We previously showed that myocardial infarction-induced microRNA-enriched extracellular vesicles (EVs) contribute to the reduction in antioxidant enzymes by targeting Nrf2 signaling in CHF. MicroRNA-27a (miRNA-27a) is the predominant microRNA contained in cardiac fibroblast-derived EVs contributing to oxidative stress along with hypertrophic gene expression in cardiomyocytes. In the present study, we observed that miRNA-27a passenger strand (miRNA-27a*) was markedly upregulated in the non-infarcted area of the left ventricle of rats with CHF and encapsulated into EVs and secreted into the circulation. Bioinformatic analysis revealed that PDZ and LIM domain 5 (PDLIM5) is one of the major targets of miRNA-27a*, playing a major role in cardiac structure and function, and potentially contributing to the progression of cardiac hypertrophy. Our in vivo data demonstrate that PDLIM5 is down-regulated in the progression of heart failure, accompanied with the upregulation of hypertrophic genes and consistent with alterations in miRNA-27a*. Moreover, exogenous administration of miRNA27a* mimics inhibit PDLIM5 translation in cardiomyocytes whereas a miRNA27a* inhibitor enhanced PDLIM5 expression. Importantly, we confirmed that infarcted hearts have higher abundance of miRNA-27a* in EVs compared to normal hearts and further demonstrated that cultured cardiac fibroblasts secrete miRNA27a*-enriched EVs into the extracellular space in response to Angiotensin II stimulation, which inhibited PDLIM5 translation, leading to cardiomyocyte hypertrophic gene expression. In vivo studies suggest that the administration of a miRNA-27a* inhibitor in CHF rats partially blocks endogenous miR-27a* expression, prevents hypertrophic gene expression and improves myocardial contractility. These findings suggest that cardiac fibroblast-secretion of miRNA27a*-enriched EVs may act as a paracrine signaling mediator of cardiac hypertrophy that has potential as a novel therapeutic target.
中文翻译:

细胞外水泡MicroRNA-27a *有助于慢性心力衰竭的心脏肥大。
在压力下,心脏会经历广泛的重塑,从而导致心脏纤维化和肥大,最终导致慢性心力衰竭(CHF)。microRNA水平的改变与心力衰竭发病机制中涉及的功能失调的基因表达谱有关。我们以前显示心肌梗死诱导的富含microRNA的细胞外囊泡(EV)通过靶向CHF中的Nrf2信号传导来减少抗氧化酶。MicroRNA-27a(miRNA-27a)是源自心脏成纤维细胞的EV中所含的主要microRNA,它们会导致氧化应激以及心肌细胞中的肥大基因表达。在目前的研究中,我们观察到,miRNA-27a过客链(miRNA-27a *)在CHF大鼠左心室的非梗塞区域显着上调,并被包埋在EV中并分泌到循环系统中。生物信息学分析表明,PDZ和LIM结构域5(PDLIM5)是miRNA-27a *的主要靶标之一,在心脏结构和功能中起着重要作用,并可能促进心脏肥大的进展。我们的体内数据表明,PDLIM5在心力衰竭的发展过程中被下调,并伴有肥大基因的上调并与miRNA-27a *的改变相一致。此外,miRNA27a *模拟物的外源给药抑制了心肌细胞中PDLIM5的翻译,而miRNA27a *抑制剂则增强了PDLIM5的表达。重要的,我们证实,与正常心脏相比,梗死的心脏在EV中具有更高的miRNA-27a *丰度,并进一步证明,培养的心脏成纤维细胞可响应血管紧张素II刺激而将富含miRNA27a *的EV分泌到细胞外空间,从而抑制PDLIM5翻译,从而导致心肌肥大基因表达。体内研究表明,在CHF大鼠中施用miRNA-27a *抑制剂可部分阻断内源性miR-27a *表达,防止肥大基因表达并改善心肌收缩力。这些发现表明,富含miRNA27a *的EV的心脏成纤维细胞分泌可能是心脏肥大的旁分泌信号传导介质,具有作为新型治疗靶标的潜力。
更新日期:2020-05-01
中文翻译:

细胞外水泡MicroRNA-27a *有助于慢性心力衰竭的心脏肥大。
在压力下,心脏会经历广泛的重塑,从而导致心脏纤维化和肥大,最终导致慢性心力衰竭(CHF)。microRNA水平的改变与心力衰竭发病机制中涉及的功能失调的基因表达谱有关。我们以前显示心肌梗死诱导的富含microRNA的细胞外囊泡(EV)通过靶向CHF中的Nrf2信号传导来减少抗氧化酶。MicroRNA-27a(miRNA-27a)是源自心脏成纤维细胞的EV中所含的主要microRNA,它们会导致氧化应激以及心肌细胞中的肥大基因表达。在目前的研究中,我们观察到,miRNA-27a过客链(miRNA-27a *)在CHF大鼠左心室的非梗塞区域显着上调,并被包埋在EV中并分泌到循环系统中。生物信息学分析表明,PDZ和LIM结构域5(PDLIM5)是miRNA-27a *的主要靶标之一,在心脏结构和功能中起着重要作用,并可能促进心脏肥大的进展。我们的体内数据表明,PDLIM5在心力衰竭的发展过程中被下调,并伴有肥大基因的上调并与miRNA-27a *的改变相一致。此外,miRNA27a *模拟物的外源给药抑制了心肌细胞中PDLIM5的翻译,而miRNA27a *抑制剂则增强了PDLIM5的表达。重要的,我们证实,与正常心脏相比,梗死的心脏在EV中具有更高的miRNA-27a *丰度,并进一步证明,培养的心脏成纤维细胞可响应血管紧张素II刺激而将富含miRNA27a *的EV分泌到细胞外空间,从而抑制PDLIM5翻译,从而导致心肌肥大基因表达。体内研究表明,在CHF大鼠中施用miRNA-27a *抑制剂可部分阻断内源性miR-27a *表达,防止肥大基因表达并改善心肌收缩力。这些发现表明,富含miRNA27a *的EV的心脏成纤维细胞分泌可能是心脏肥大的旁分泌信号传导介质,具有作为新型治疗靶标的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号