当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of disulfide (cystine) oxidation by HOCl in a model peptide: Evidence for oxygen addition, disulfide bond cleavage and adduct formation with thiols.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.freeradbiomed.2020.04.023 Maryam Karimi 1 , Ben Crossett 2 , Stuart J Cordwell 3 , David I Pattison 1 , Michael J Davies 4
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.freeradbiomed.2020.04.023 Maryam Karimi 1 , Ben Crossett 2 , Stuart J Cordwell 3 , David I Pattison 1 , Michael J Davies 4
Affiliation
|
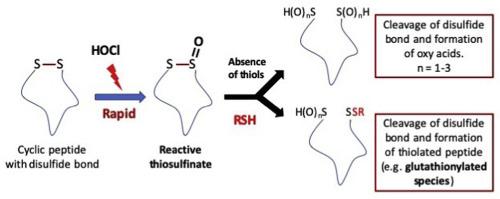
Disulfide bonds play a key role in stabilizing proteins by cross-linking secondary structures. Whilst many disulfides are effectively unreactive, it is increasingly clear that some disulfides are redox active, participate in enzymatic reactions and/or regulate protein function by allosteric mechanisms. Previously (Karimi et al., Sci. Rep. 2016, 6, 38752) we have shown that some disulfides react rapidly with biological oxidants due to favourable interactions with available lone-pairs of electrons. Here we present data from kinetic, mechanistic and product studies for HOCl-mediated oxidation of a protected nine-amino acid model peptide containing a N- to C-terminal disulfide bond. This peptide reacts with HOCl with k2 1.8 × 106 M-1 s-1, similar to other highly-reactive disulfide-containing compounds. With low oxidant excesses, oxidation yields multiple oxidation products from the disulfide, with reaction predominating at the N-terminal Cys to give sulfenic, sulfinic and sulfonic acids, and disulfide bond cleavage. Limited oxidation occurs, with higher oxidant excesses, at Trp and His residues to give mono- and di- (for Trp) oxygenated products. Site-specific backbone cleavage also occurs between Arg and Trp, probably via initial side-chain modification. Treatment of the previously-oxidised peptide with thiols (GSH, N-Ac-Cys), results in adduction of the thiol to the oxidised peptide, with this occurring at the original disulfide bond. This gives an open-chain peptide, and a new mixed disulfide containing GSH or N-Ac-Cys as determined by mass spectrometry. Disulfide bond oxidation may therefore markedly alter the structure, activity and function of disulfide-containing proteins, and provides a potential mechanism for protein glutathionylation.
中文翻译:

模型肽中HOCl对二硫键(胱氨酸)的氧化特性:氧加成,二硫键断裂和与硫醇形成加合物的证据。
二硫键通过交联二级结构在稳定蛋白质中起关键作用。尽管许多二硫化物实际上没有反应性,但越来越清楚的是,某些二硫化物具有氧化还原活性,通过变构机制参与酶促反应和/或调节蛋白质功能。以前(Karimi et al。,Sci。Rep。2016,6,38752),我们已经表明,由于与可用电子孤对的良好相互作用,一些二硫化物与生物氧化剂迅速反应。在这里,我们介绍了从HOCl介导的包含N端至C端二硫键的受保护九氨基酸模型肽的HOCl介导的氧化反应的动力学,机理和产物研究数据。该肽与kCl为1.8×106 M-1 s-1的HOCl反应,类似于其他高反应性的含二硫化物的化合物。低氧化剂过量,氧化产生了来自二硫化物的多种氧化产物,反应主要在N端Cys上进行,从而产生亚磺酸,亚磺酸和磺酸,以及二硫键断裂。在Trp和His残基处发生有限的氧化,并带有较高的氧化剂过量,生成单和双(对于Trp)氧化的产物。特定位点的骨架裂解也可能发生在Arg和Trp之间,可能是通过最初的侧链修饰。用硫醇(GSH,N-Ac-Cys)处理先前氧化的肽,导致硫醇加成到氧化的肽上,这发生在原始的二硫键上。这样就得到了一个开链肽,以及一种新的含有GSH或N-Ac-Cys的混合二硫化物(通过质谱法测定)。因此,二硫键氧化可能会显着改变结构,
更新日期:2020-05-01
中文翻译:

模型肽中HOCl对二硫键(胱氨酸)的氧化特性:氧加成,二硫键断裂和与硫醇形成加合物的证据。
二硫键通过交联二级结构在稳定蛋白质中起关键作用。尽管许多二硫化物实际上没有反应性,但越来越清楚的是,某些二硫化物具有氧化还原活性,通过变构机制参与酶促反应和/或调节蛋白质功能。以前(Karimi et al。,Sci。Rep。2016,6,38752),我们已经表明,由于与可用电子孤对的良好相互作用,一些二硫化物与生物氧化剂迅速反应。在这里,我们介绍了从HOCl介导的包含N端至C端二硫键的受保护九氨基酸模型肽的HOCl介导的氧化反应的动力学,机理和产物研究数据。该肽与kCl为1.8×106 M-1 s-1的HOCl反应,类似于其他高反应性的含二硫化物的化合物。低氧化剂过量,氧化产生了来自二硫化物的多种氧化产物,反应主要在N端Cys上进行,从而产生亚磺酸,亚磺酸和磺酸,以及二硫键断裂。在Trp和His残基处发生有限的氧化,并带有较高的氧化剂过量,生成单和双(对于Trp)氧化的产物。特定位点的骨架裂解也可能发生在Arg和Trp之间,可能是通过最初的侧链修饰。用硫醇(GSH,N-Ac-Cys)处理先前氧化的肽,导致硫醇加成到氧化的肽上,这发生在原始的二硫键上。这样就得到了一个开链肽,以及一种新的含有GSH或N-Ac-Cys的混合二硫化物(通过质谱法测定)。因此,二硫键氧化可能会显着改变结构,











































 京公网安备 11010802027423号
京公网安备 11010802027423号