Pharmacology Biochemistry and Behavior ( IF 3.6 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.pbb.2020.172933 Solenn Percelay 1 , Marc Since 2 , Stéphanie Lagadu 3 , Thomas Freret 1 , Valentine Bouet 1 , Michel Boulouard 1
|
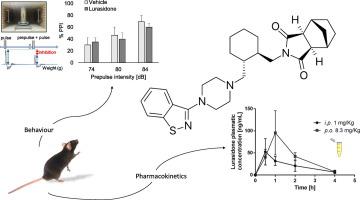
Lurasidone is an atypical antipsychotic that has been shown to be effective in reversing schizophrenia-related cognitive impairment. The development of new preclinical models of schizophrenia is a key for improving treatments of cognitive symptoms. This study investigated the effects of chronic lurasidone treatment in C57BL/6 male mice via intraperitoneal injection (1 mg/kg daily at 5 p.m. for 5 weeks). A large battery of behavioural tests was performed (between 9 a.m. and 5 p.m.), which is currently used to assess face validity in animal models of psychiatric diseases. Overall, lurasidone did not interfere with behavioural performances, which characterises very good tolerance to such a high dose. Moreover, pharmacokinetic parameters after i.p. and oral administration were measured. Mean transit time (MTT) values were 1.91 h (1 mg/kg acute i.p.) and 1.74 h (8.3 mg/kg acute oral), respectively, and relative bioavailability comparing these two routes of administration was of 19.8%. This last result gives important data to adapt oral chronic administration of lurasidone with a more ethical perspective in comparison with chronic i.p. injections. This study brings tools to improve pharmacological validity of preclinical models of psychiatric diseases, and to adapt dosage of antipsychotics according to the route used.
中文翻译:

抗精神病药卢拉西酮:C57BL / 6小鼠的行为和药代动力学数据。
卢拉西酮是一种非典型的抗精神病药,已被证明可有效逆转精神分裂症相关的认知障碍。新的精神分裂症临床前模型的开发是改善认知症状治疗的关键。这项研究调查了慢性路拉西酮对C57BL / 6雄性小鼠的治疗作用,方法是通过腹膜内注射(每天1 mg / kg,每天下午5点,持续5周)。进行了大量的行为测试(上午9点至下午5点之间),目前用于评估精神疾病动物模型中的面部有效性。总体而言,卢拉西酮不干扰行为表现,这表现出对如此高剂量的很好耐受性。而且,测量了腹膜内和口服给药后的药代动力学参数。平均通过时间(MTT)值分别为1.91小时(1 mg / kg急性腹膜内)和1.74小时(8.3 mg / kg急性口服),比较这两种给药途径的相对生物利用度为19.8%。最后的结果提供了重要的数据,与慢性腹膜内注射相比,它具有更符合伦理学的观点,可适应口服长期口服卢拉西酮。



























 京公网安备 11010802027423号
京公网安备 11010802027423号