当前位置:
X-MOL 学术
›
BBA Mol. Cell Biol. Lipids
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lysosomal acid lipase is the major acid retinyl ester hydrolase in cultured human hepatic stellate cells but not essential for retinyl ester degradation.
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids ( IF 3.9 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.bbalip.2020.158730 Carina Wagner 1 , Victoria Hois 1 , Laura Pajed 1 , Lisa-Maria Pusch 1 , Heimo Wolinski 1 , Michael Trauner 2 , Robert Zimmermann 3 , Ulrike Taschler 1 , Achim Lass 3
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids ( IF 3.9 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.bbalip.2020.158730 Carina Wagner 1 , Victoria Hois 1 , Laura Pajed 1 , Lisa-Maria Pusch 1 , Heimo Wolinski 1 , Michael Trauner 2 , Robert Zimmermann 3 , Ulrike Taschler 1 , Achim Lass 3
Affiliation
|
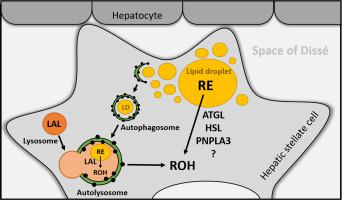
Vitamin A is stored as retinyl esters (REs) in lipid droplets of hepatic stellate cells (HSCs). To date, two different pathways are known to facilitate the breakdown of REs: (i) Hydrolysis of REs by neutral lipases, and (ii) whole lipid droplet degradation in autolysosomes by acid hydrolysis. In this study, we evaluated the contribution of neutral and acid RE hydrolases to the breakdown of REs in human HSCs. (R)-Bromoenol lactone (R-BEL), inhibitor of adipose triglyceride lipase (ATGL) and patatin-like phospholipase domain-containing 3 (PNPLA3), the hormone-sensitive lipase (HSL) inhibitor 76-0079, as well as the serine-hydrolase inhibitor Orlistat reduced neutral RE hydrolase activity of LX-2 cell-lysates between 20 and 50%. Interestingly, in pulse-chase experiments, R-BEL, 76-0079, as well as Orlistat exerted little to no effect on cellular RE breakdown of LX-2 cells as well as primary human HSCs. In contrast, Lalistat2, a specific lysosomal acid lipase (LAL) inhibitor, virtually blunted acid in vitro RE hydrolase activity of LX-2 cells. Accordingly, HSCs isolated from LAL-deficient mice showed RE accumulation and were virtually devoid of acidic RE hydrolase activity. In pulse-chase experiments however, LAL-deficient HSCs, similar to LX-2 cells and primary human HSCs, were not defective in degrading REs. In summary, results demonstrate that ATGL, PNPLA3, and HSL contribute to neutral RE hydrolysis of human HSCs. LAL is the major acid RE hydrolase in HSCs. Yet, LAL is not limiting for RE degradation under serum-starvation. Together, results suggest that RE breakdown of HSCs is facilitated by (a) so far unknown, non-Orlistat inhibitable RE-hydrolase(s).
中文翻译:

溶酶体酸性脂肪酶是培养的人肝星状细胞中主要的酸性视黄酯水解酶,但对视黄酯降解不是必需的。
维生素 A 以视黄酯 (RE) 的形式储存在肝星状细胞 (HSC) 的脂滴中。迄今为止,已知有两种不同的途径可以促进 RE 的分解:(i)中性脂肪酶对 RE 的水解,和(ii)通过酸水解在自溶酶体中降解整个脂滴。在这项研究中,我们评估了中性和酸性 RE 水解酶对人类 HSC 中 RE 分解的贡献。(R)-溴烯醇内酯 (R-BEL),脂肪甘油三酯脂肪酶 (ATGL) 和含有 patatin 样磷脂酶结构域 3 (PNPLA3) 的抑制剂,激素敏感性脂肪酶 (HSL) 抑制剂 76-0079,以及丝氨酸水解酶抑制剂奥利司他将 LX-2 细胞裂解物的中性 RE 水解酶活性降低 20% 至 50%。有趣的是,在脉冲追踪实验中,R-BEL,76-0079,以及奥利司他对 LX-2 细胞以及原代人类 HSC 的细胞 RE 分解几乎没有影响。相比之下,特定的溶酶体酸性脂肪酶 (LAL) 抑制剂 Lalistat2 几乎减弱了 LX-2 细胞的体外 RE 水解酶活性。因此,从 LAL 缺陷小鼠中分离的 HSC 显示出 RE 积累,并且几乎没有酸性 RE 水解酶活性。然而,在脉冲追踪实验中,LAL 缺陷型 HSC,类似于 LX-2 细胞和原代人类 HSC,在降解 RE 方面没有缺陷。总之,结果表明 ATGL、PNPLA3 和 HSL 有助于人类 HSC 的中性 RE 水解。LAL 是 HSC 中主要的酸性 RE 水解酶。然而,LAL 并不限制血清饥饿下的 RE 降解。总之,结果表明 HSC 的 RE 分解是由(a)迄今为止未知的,
更新日期:2020-05-01
中文翻译:

溶酶体酸性脂肪酶是培养的人肝星状细胞中主要的酸性视黄酯水解酶,但对视黄酯降解不是必需的。
维生素 A 以视黄酯 (RE) 的形式储存在肝星状细胞 (HSC) 的脂滴中。迄今为止,已知有两种不同的途径可以促进 RE 的分解:(i)中性脂肪酶对 RE 的水解,和(ii)通过酸水解在自溶酶体中降解整个脂滴。在这项研究中,我们评估了中性和酸性 RE 水解酶对人类 HSC 中 RE 分解的贡献。(R)-溴烯醇内酯 (R-BEL),脂肪甘油三酯脂肪酶 (ATGL) 和含有 patatin 样磷脂酶结构域 3 (PNPLA3) 的抑制剂,激素敏感性脂肪酶 (HSL) 抑制剂 76-0079,以及丝氨酸水解酶抑制剂奥利司他将 LX-2 细胞裂解物的中性 RE 水解酶活性降低 20% 至 50%。有趣的是,在脉冲追踪实验中,R-BEL,76-0079,以及奥利司他对 LX-2 细胞以及原代人类 HSC 的细胞 RE 分解几乎没有影响。相比之下,特定的溶酶体酸性脂肪酶 (LAL) 抑制剂 Lalistat2 几乎减弱了 LX-2 细胞的体外 RE 水解酶活性。因此,从 LAL 缺陷小鼠中分离的 HSC 显示出 RE 积累,并且几乎没有酸性 RE 水解酶活性。然而,在脉冲追踪实验中,LAL 缺陷型 HSC,类似于 LX-2 细胞和原代人类 HSC,在降解 RE 方面没有缺陷。总之,结果表明 ATGL、PNPLA3 和 HSL 有助于人类 HSC 的中性 RE 水解。LAL 是 HSC 中主要的酸性 RE 水解酶。然而,LAL 并不限制血清饥饿下的 RE 降解。总之,结果表明 HSC 的 RE 分解是由(a)迄今为止未知的,











































 京公网安备 11010802027423号
京公网安备 11010802027423号