当前位置:
X-MOL 学术
›
Genes Cells
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Homeodomain protein DLX4 facilitates nasopharyngeal carcinoma progression via up-regulation of YB-1.
Genes to Cells ( IF 2.1 ) Pub Date : 2020-04-12 , DOI: 10.1111/gtc.12772 Zeyi Ling 1 , Xiaoli Long 2 , Jie Li 1 , Mingliang Feng 1
Genes to Cells ( IF 2.1 ) Pub Date : 2020-04-12 , DOI: 10.1111/gtc.12772 Zeyi Ling 1 , Xiaoli Long 2 , Jie Li 1 , Mingliang Feng 1
Affiliation
|
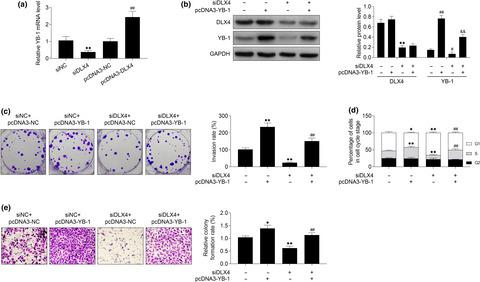
Nasopharyngeal carcinoma (NPC) is a malignant tumor in nasopharynx tissues and lacks effective treatment strategies. Dysregulation of distal‐less homeobox 4 (DLX4) participates in the development of tumors. Understanding the regulatory mechanism of DLX4 in NPC progression may address this issue. Here, we first identified an up‐regulation of DLX4 in NPC cell lines compared to normal epithelial cells. Data from colony formation and transwell assays showed that knockdown of DLX4 inhibited cell proliferation and invasion of NPC, respectively. Moreover, DLX4 knockdown blocked the cell cycle of NPC at G1 phase, suggesting the antitumor effect of DLX4 knockdown on NPC. The downstream target of DLX4 was identified as Y‐box binding protein 1 (YB‐1), whose expression was increased by over‐expression of DLX4, while decreased by knockdown of DLX4. The binding capacity between DLX4 and YB‐1 was verified by chromatin immunoprecipitation (ChIP), and the result showed that DLX4 could not directly bind to the promoter of YB‐1. Mechanically, YB‐1 over‐expression reversed the effects of DLX4 knockdown on cell proliferation, cell cycle arrest and cell invasion of NPC. In conclusion, our findings indicated that DLX4 promoted NPC progression via up‐regulation of YB‐1, which would shed light on therapeutic schedule in NPC.
中文翻译:

同源结构域蛋白DLX4通过上调YB-1促进鼻咽癌的进展。
鼻咽癌(NPC)是鼻咽组织中的一种恶性肿瘤,缺乏有效的治疗策略。无远端同源盒4(DLX4)的异常调节参与了肿瘤的发展。了解DLX4在NPC进展中的调控机制可能会解决此问题。在这里,我们首先发现与正常上皮细胞相比,NPC细胞系中的DLX4上调。集落形成和transwell测定的数据表明,敲低DLX4分别抑制细胞增殖和NPC侵袭。此外,DLX4敲低阻断了NPC在G1期的细胞周期,提示DLX4敲低对NPC的抗肿瘤作用。DLX4的下游靶标被鉴定为Y-box结合蛋白1(YB-1),其表达由于DLX4的过表达而增加,而由于DLX4的敲低而降低。通过染色质免疫沉淀(ChIP)验证了DLX4与YB-1之间的结合能力,结果表明DLX4无法直接与YB-1的启动子结合。在机械上,YB-1过表达逆转了DLX4敲低对NPC细胞增殖,细胞周期停滞和细胞侵袭的影响。总之,我们的发现表明DLX4通过上调YB-1促进了NPC的进展,这将阐明NPC的治疗方案。
更新日期:2020-04-12
中文翻译:

同源结构域蛋白DLX4通过上调YB-1促进鼻咽癌的进展。
鼻咽癌(NPC)是鼻咽组织中的一种恶性肿瘤,缺乏有效的治疗策略。无远端同源盒4(DLX4)的异常调节参与了肿瘤的发展。了解DLX4在NPC进展中的调控机制可能会解决此问题。在这里,我们首先发现与正常上皮细胞相比,NPC细胞系中的DLX4上调。集落形成和transwell测定的数据表明,敲低DLX4分别抑制细胞增殖和NPC侵袭。此外,DLX4敲低阻断了NPC在G1期的细胞周期,提示DLX4敲低对NPC的抗肿瘤作用。DLX4的下游靶标被鉴定为Y-box结合蛋白1(YB-1),其表达由于DLX4的过表达而增加,而由于DLX4的敲低而降低。通过染色质免疫沉淀(ChIP)验证了DLX4与YB-1之间的结合能力,结果表明DLX4无法直接与YB-1的启动子结合。在机械上,YB-1过表达逆转了DLX4敲低对NPC细胞增殖,细胞周期停滞和细胞侵袭的影响。总之,我们的发现表明DLX4通过上调YB-1促进了NPC的进展,这将阐明NPC的治疗方案。



























 京公网安备 11010802027423号
京公网安备 11010802027423号