当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper(II) partially protects three histidine residues and the N-terminus of amyloid-β peptide from diethyl pyrocarbonate (DEPC) modification.
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-04-29 , DOI: 10.1002/2211-5463.12857 Merlin Friedemann 1 , Vello Tõugu 1 , Peep Palumaa 1
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-04-29 , DOI: 10.1002/2211-5463.12857 Merlin Friedemann 1 , Vello Tõugu 1 , Peep Palumaa 1
Affiliation
|
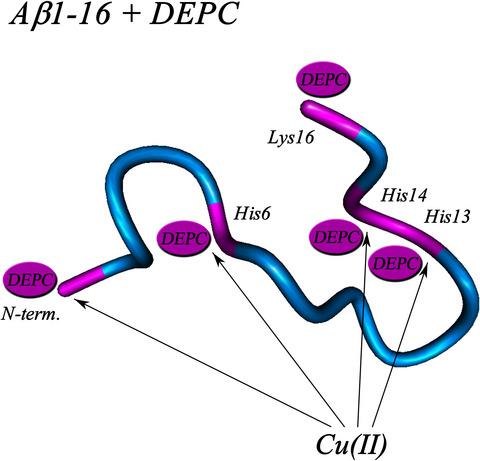
Diethyl pyrocarbonate (DEPC) has been primarily used as a residue‐specific modifying agent to study the role of His residues in peptide/protein and enzyme function; however, its action is not specific, and several other residues can also be modified. In the current study, we monitored the reaction of DEPC with amyloid‐beta (Aβ) peptides and insulin by matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry (MALDI‐TOF MS) and determined the modification sites by electrospray ionization quadrupole time‐of‐flight tandem MS (ESI Q‐TOF MS/MS). Our results indicate that five residues in Aβ1–42 are modified in the presence of 30‐fold molar excess of DEPC. After hydroxylamine treatment (which removes modifications from three His residues), two labels remain bound at the peptide N terminus and Lys16. DEPC treatment of Aβ1–42 promotes peptide aggregation, as monitored through the loss of soluble Aβ42 in a semi‐quantitative MALDI‐TOF MS assay. It has been previously proposed that Cu(II) ions protect Aβ1–16 from DEPC modification through binding to His6. We confirmed that Cu(II) ions decrease the stoichiometry of Aβ1–16 modification with the excess of DEPC being lower as compared to the control, which indicates that Cu(II) protects Aβ from DEPC modification. Sequencing of obtained Cu(II)‐protected Aβ1–16 samples showed that Cu(II) does not protect any residues completely, but partially protects all three His residues and the N terminus. Thus, the protection by Cu(II) ions is not related to specific metal binding to a particular residue (e.g. His6), but rather all His residues and the N terminus are involved in binding of Cu(II) ions. These results allow us to elucidate the effect of DEPC modification on amyloidogenity of human Aβ and to speculate about the role of His residues in these processes.
中文翻译:

铜(II)部分保护了三个组氨酸残基和淀粉样β肽的N端免受焦碳酸二乙酯(DEPC)修饰。
焦碳酸二乙酯(DEPC)主要用作残基特异性修饰剂,用于研究His残基在肽/蛋白质和酶功能中的作用;然而,它的作用不是特异性的,还可以修饰其他一些残基。在本研究中,我们通过基质辅助激光解吸/电离飞行时间质谱(MALDI-TOF MS)监测了DEPC与淀粉样β(Aβ)肽和胰岛素的反应,并通过电喷雾电离确定了修饰位点四极杆飞行时间串联质谱仪(ESI Q-TOF MS / MS)。我们的结果表明,在DEPC摩尔比过量30倍的情况下,Aβ1-42中的5个残基被修饰。羟胺处理后(可去除三个His残基的修饰),两个标记保留在肽N端和Lys16上。在半定量MALDI-TOF MS分析中,通过可溶性Aβ42的丢失监测到DEPC处理Aβ1-42会促进肽聚集。先前曾有人提出Cu(II)离子通过与His6结合来保护Aβ1-16免受DEPC修饰。我们证实,与对照相比,Cu(II)离子降低了Aβ1–16修饰的化学计量,而过量的DEPC则比对照组低,这表明Cu(II)保护了Aβ免受DEPC修饰。对获得的由Cu(II)保护的Aβ1–16样品的测序表明,Cu(II)不能完全保护任何残基,但是可以部分保护所有三个His残基和N末端。因此,Cu(II)离子的保护与特定金属与特定残基(例如His6)的结合无关,而是所有His残基和N末端均与Cu(II)离子的结合有关。
更新日期:2020-04-29
中文翻译:

铜(II)部分保护了三个组氨酸残基和淀粉样β肽的N端免受焦碳酸二乙酯(DEPC)修饰。
焦碳酸二乙酯(DEPC)主要用作残基特异性修饰剂,用于研究His残基在肽/蛋白质和酶功能中的作用;然而,它的作用不是特异性的,还可以修饰其他一些残基。在本研究中,我们通过基质辅助激光解吸/电离飞行时间质谱(MALDI-TOF MS)监测了DEPC与淀粉样β(Aβ)肽和胰岛素的反应,并通过电喷雾电离确定了修饰位点四极杆飞行时间串联质谱仪(ESI Q-TOF MS / MS)。我们的结果表明,在DEPC摩尔比过量30倍的情况下,Aβ1-42中的5个残基被修饰。羟胺处理后(可去除三个His残基的修饰),两个标记保留在肽N端和Lys16上。在半定量MALDI-TOF MS分析中,通过可溶性Aβ42的丢失监测到DEPC处理Aβ1-42会促进肽聚集。先前曾有人提出Cu(II)离子通过与His6结合来保护Aβ1-16免受DEPC修饰。我们证实,与对照相比,Cu(II)离子降低了Aβ1–16修饰的化学计量,而过量的DEPC则比对照组低,这表明Cu(II)保护了Aβ免受DEPC修饰。对获得的由Cu(II)保护的Aβ1–16样品的测序表明,Cu(II)不能完全保护任何残基,但是可以部分保护所有三个His残基和N末端。因此,Cu(II)离子的保护与特定金属与特定残基(例如His6)的结合无关,而是所有His残基和N末端均与Cu(II)离子的结合有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号