当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical investigation of an acid catalyst for viable release of H2 from BN nanotubes: A local pair natural orbital coupled cluster approach
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-04-30 , DOI: 10.1002/qua.26257 Lisa Roy 1
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-04-30 , DOI: 10.1002/qua.26257 Lisa Roy 1
Affiliation
|
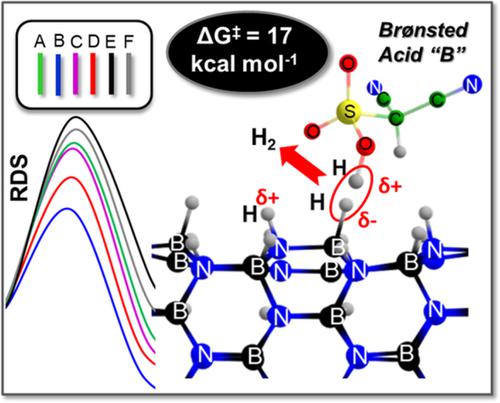
Catalytic removal of H2 from boron‐nitride (BN)‐based nanomaterials at ambient conditions is of paramount importance in order to develop lightweight hydrogen storage media. Here, the DLPNO‐CCSD(T) technique is used to calculate accurate relative energies and activation barriers of Brønsted acid‐initiated removal of H2 from hydrogenated BN nanotubes (HBNNTs) with six different acids. Three crucial steps are identified in the mechanism: first H2 release, catalyst regeneration via proton transfer, and second H2 release to ensure feasibility of the dehydrogenation proposal. Our computational studies reveal that sulfonic acids with appropriate electrophilicity can facilitate dehydrogenation of HBNNT at a low free energetic cost (∆G‡ = 17 kcal/mol). Importantly, these findings illustrate the possibility of H2 release from BN nanomaterials at ambient conditions and provides hope for a sustainable chemical hydrogen storage strategy.
中文翻译:

用于从BN纳米管中释放H2的酸催化剂的理论研究:局部对自然轨道耦合簇方法
为了开发轻质的氢存储介质,在环境条件下从氮化硼(BN)基纳米材料中催化去除H 2至关重要。在这里,DLPNO-CCSD(T)技术用于计算准确的相对能量和布朗斯台德酸引发的用六种不同酸从氢化BN纳米管(HBNNTs)中去除H 2的活化势垒。该机理中确定了三个关键步骤:第一个H 2释放,通过质子转移的催化剂再生和第二个H 2释放,以确保脱氢方案的可行性。我们的计算研究表明,具有适当亲电性的磺酸可以以较低的自由能成本(∆G‡ = 17 kcal / mol)。重要的是,这些发现说明了在环境条件下H 2从BN纳米材料释放的可能性,并为可持续的化学氢存储策略提供了希望。
更新日期:2020-04-30
中文翻译:

用于从BN纳米管中释放H2的酸催化剂的理论研究:局部对自然轨道耦合簇方法
为了开发轻质的氢存储介质,在环境条件下从氮化硼(BN)基纳米材料中催化去除H 2至关重要。在这里,DLPNO-CCSD(T)技术用于计算准确的相对能量和布朗斯台德酸引发的用六种不同酸从氢化BN纳米管(HBNNTs)中去除H 2的活化势垒。该机理中确定了三个关键步骤:第一个H 2释放,通过质子转移的催化剂再生和第二个H 2释放,以确保脱氢方案的可行性。我们的计算研究表明,具有适当亲电性的磺酸可以以较低的自由能成本(∆G‡ = 17 kcal / mol)。重要的是,这些发现说明了在环境条件下H 2从BN纳米材料释放的可能性,并为可持续的化学氢存储策略提供了希望。











































 京公网安备 11010802027423号
京公网安备 11010802027423号