当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and Synthesis of Dihydroxamic Acids as HDAC6/8/10 Inhibitors.
ChemMedChem ( IF 3.6 ) Pub Date : 2020-04-29 , DOI: 10.1002/cmdc.202000149 Michael Morgen 1 , Raphael R Steimbach 1, 2 , Magalie Géraldy 1 , Lars Hellweg 1 , Peter Sehr 3 , Johannes Ridinger 4, 5, 6 , Olaf Witt 4, 5, 6, 7 , Ina Oehme 4, 5, 6 , Corey J Herbst-Gervasoni 8 , Jeremy D Osko 8 , Nicholas J Porter 8 , David W Christianson 8 , Nikolas Gunkel 1, 7 , Aubry K Miller 1, 7
ChemMedChem ( IF 3.6 ) Pub Date : 2020-04-29 , DOI: 10.1002/cmdc.202000149 Michael Morgen 1 , Raphael R Steimbach 1, 2 , Magalie Géraldy 1 , Lars Hellweg 1 , Peter Sehr 3 , Johannes Ridinger 4, 5, 6 , Olaf Witt 4, 5, 6, 7 , Ina Oehme 4, 5, 6 , Corey J Herbst-Gervasoni 8 , Jeremy D Osko 8 , Nicholas J Porter 8 , David W Christianson 8 , Nikolas Gunkel 1, 7 , Aubry K Miller 1, 7
Affiliation
|
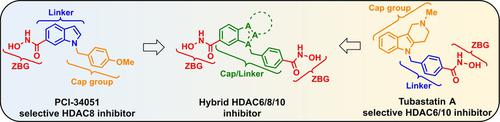
We report the synthesis and evaluation of a class of selective multitarget agents for the inhibition of HDAC6, HDAC8, and HDAC10. The concept for this study grew out of a structural analysis of the two selective inhibitors Tubastatin A (HDAC6/10) and PCI‐34051 (HDAC8), which we recognized share the same N‐benzylindole core. Hybridization of the two inhibitor structures resulted in dihydroxamic acids with benzyl‐indole and ‐indazole core motifs. These substances exhibit potent activity against HDAC6, HDAC8, and HDAC10, while retaining selectivity over HDAC1, HDAC2, and HDAC3. The best substance inhibited the viability of the SK‐N‐BE(2)C neuroblastoma cell line with an IC50 value similar to a combination treatment with Tubastatin A and PCI‐34051. This compound class establishes a proof of concept for such hybrid molecules and could serve as a starting point for the further development of enhanced HDAC6/8/10 inhibitors.
中文翻译:

作为 HDAC6/8/10 抑制剂的二异羟肟酸的设计和合成。
我们报告了一类用于抑制 HDAC6、HDAC8 和 HDAC10 的选择性多靶点药物的合成和评估。这项研究的概念源于对两种选择性抑制剂图巴他汀 A (HDAC6/10) 和 PCI-34051 (HDAC8) 的结构分析,我们认识到它们具有相同的N-苄吲哚核心。两种抑制剂结构的杂交产生了具有苄基吲哚和吲唑核心基序的二异羟肟酸。这些物质对 HDAC6、HDAC8 和 HDAC10 表现出有效的活性,同时保留对 HDAC1、HDAC2 和 HDAC3 的选择性。最好的物质抑制 SK-N-BE(2)C 神经母细胞瘤细胞系的活力,其 IC 50值与图巴他汀 A 和 PCI-34051 的联合治疗相似。该化合物类别为此类混合分子建立了概念验证,并可作为进一步开发增强型 HDAC6/8/10 抑制剂的起点。
更新日期:2020-07-03
中文翻译:

作为 HDAC6/8/10 抑制剂的二异羟肟酸的设计和合成。
我们报告了一类用于抑制 HDAC6、HDAC8 和 HDAC10 的选择性多靶点药物的合成和评估。这项研究的概念源于对两种选择性抑制剂图巴他汀 A (HDAC6/10) 和 PCI-34051 (HDAC8) 的结构分析,我们认识到它们具有相同的N-苄吲哚核心。两种抑制剂结构的杂交产生了具有苄基吲哚和吲唑核心基序的二异羟肟酸。这些物质对 HDAC6、HDAC8 和 HDAC10 表现出有效的活性,同时保留对 HDAC1、HDAC2 和 HDAC3 的选择性。最好的物质抑制 SK-N-BE(2)C 神经母细胞瘤细胞系的活力,其 IC 50值与图巴他汀 A 和 PCI-34051 的联合治疗相似。该化合物类别为此类混合分子建立了概念验证,并可作为进一步开发增强型 HDAC6/8/10 抑制剂的起点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号