当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A molecular dynamics approach on the Y393C variant of protein disulfide isomerase A1.
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2020-04-30 , DOI: 10.1111/cbdd.13700 Pablo A Madero-Ayala 1 , Rosa E Mares-Alejandre 1 , Marco A Ramos-Ibarra 1
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2020-04-30 , DOI: 10.1111/cbdd.13700 Pablo A Madero-Ayala 1 , Rosa E Mares-Alejandre 1 , Marco A Ramos-Ibarra 1
Affiliation
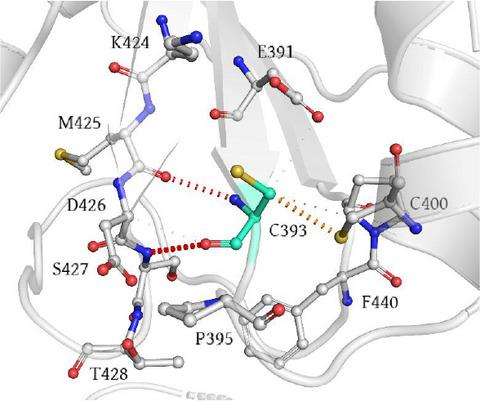
|
Human protein disulfide isomerase A1 (PDIA1) shows both catalytic (i.e., oxidoreductase) and non‐catalytic (i.e., chaperone) activities and plays a crucial role in the oxidative folding of proteins within the endoplasmic reticulum. PDIA1 dysregulation is a common trait in numerous pathophysiological conditions, including neurodegenerative disorders and cancerous diseases. The 1178A>G mutation of the human PDIA1‐encoding gene is a non‐synonymous single nucleotide polymorphism detected in patients with Cole‐Carpenter syndrome type 1 (CSS1), a particularly rare bone disease. In vitro studies showed that the encoded variant (PDIA1 Y393C) exhibits limited oxidoreductase activity. To gain knowledge on the structure–function relationship, we undertook a molecular dynamics (MD) approach to examine the structural stability of PDIA1 Y393C. Results showed that significant conformational changes are the structural consequence of the amino acid substitution Tyr>Cys at position 393 of the PDIA1 protein. This structure‐based study provides further knowledge about the molecular origin of CCS1.
中文翻译:

蛋白质二硫键异构酶 A1 的 Y393C 变体的分子动力学方法。
人类蛋白质二硫化物异构酶 A1 (PDIA1) 显示出催化(即氧化还原酶)和非催化(即分子伴侣)活性,并且在内质网内蛋白质的氧化折叠中起关键作用。PDIA1 失调是许多病理生理疾病的常见特征,包括神经退行性疾病和癌症疾病。人类 PDIA1 编码基因的 1178A>G 突变是在 Cole-Carpenter 综合征 1 型(CSS1)患者中检测到的一种非同义单核苷酸多态性,这是一种特别罕见的骨病。体外研究表明,编码的变体 (PDIA1 Y393C) 表现出有限的氧化还原酶活性。为了了解结构-功能关系,我们采用分子动力学 (MD) 方法来检查 PDIA1 Y393C 的结构稳定性。结果表明,显着的构象变化是 PDIA1 蛋白第 393 位氨基酸取代 Tyr>Cys 的结构结果。这项基于结构的研究提供了关于 CCS1 分子起源的进一步知识。
更新日期:2020-04-30
中文翻译:

蛋白质二硫键异构酶 A1 的 Y393C 变体的分子动力学方法。
人类蛋白质二硫化物异构酶 A1 (PDIA1) 显示出催化(即氧化还原酶)和非催化(即分子伴侣)活性,并且在内质网内蛋白质的氧化折叠中起关键作用。PDIA1 失调是许多病理生理疾病的常见特征,包括神经退行性疾病和癌症疾病。人类 PDIA1 编码基因的 1178A>G 突变是在 Cole-Carpenter 综合征 1 型(CSS1)患者中检测到的一种非同义单核苷酸多态性,这是一种特别罕见的骨病。体外研究表明,编码的变体 (PDIA1 Y393C) 表现出有限的氧化还原酶活性。为了了解结构-功能关系,我们采用分子动力学 (MD) 方法来检查 PDIA1 Y393C 的结构稳定性。结果表明,显着的构象变化是 PDIA1 蛋白第 393 位氨基酸取代 Tyr>Cys 的结构结果。这项基于结构的研究提供了关于 CCS1 分子起源的进一步知识。



























 京公网安备 11010802027423号
京公网安备 11010802027423号