当前位置:
X-MOL 学术
›
Biotechnol. Appl. Bioc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cloning, characterization, and enzymatic identification of a new tryptophan decarboxylase from Ophiorrhiza pumila
Biotechnology and Applied Biochemistry ( IF 3.2 ) Pub Date : 2020-04-30 , DOI: 10.1002/bab.1935 Dawei You 1, 2 , Yue Feng 2 , Can Wang 2 , Chengtao Sun 2 , Yao Wang 2 , Degang Zhao 1 , Guoyin Kai 2
Biotechnology and Applied Biochemistry ( IF 3.2 ) Pub Date : 2020-04-30 , DOI: 10.1002/bab.1935 Dawei You 1, 2 , Yue Feng 2 , Can Wang 2 , Chengtao Sun 2 , Yao Wang 2 , Degang Zhao 1 , Guoyin Kai 2
Affiliation
|
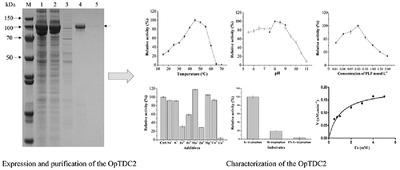
Tryptophan decarboxylase (TDC, EC 4.1.1.28) catalyzes tryptophan decarboxylation to form tryptamine through the cofactor pyridoxal‐5′‐phosphate (PLP), a crucial stage in the production of the terpenoid indole alkaloids like camptothecin (CPT). A new gene encoding TDC was identified from the CPT‐producing plant Ophiorrhiza pumila by transcriptome analysis, termed OpTDC2. It contained a 1,536 bp open reading frame that encodes a 511 amino acid protein with a molecular mass of 57.01 kDa and an isoelectric point of 6.39. Multiple sequence alignment and phylogenetic tree analysis showed the closest similarity (85%) with the TDC from Mitragyna speciosa. Moreover, the highest expression of OpTDC2 was observed in the O. pumila root. To achieve high‐efficiency expression of OpTDC2 in Escherichia coli, we fused the TF tag onto the N‐terminal of the OpTDC2. Optimum enzymatic activity was observed at 45 °C, pH 8 and cofactor concentration of 0.1 mM. The catalytic reaction was strongly inhibited by metal ions of Cu2+, Zn2+, and Fe2+. The l‐tryptophan was particularly catalyzed compared with d‐tryptophan. Besides, the Km and kcat of the OpTDC2 were 1.08 mM and 0.78 Sec−1, respectively. The results provided information on new functional OpTDC2 that might be used in synthetic biology for the enhanced biosynthesis of CPT in O. pumila.
中文翻译:

一种来自Ophiorrhiza pumila的新型色氨酸脱羧酶的克隆,表征和酶促鉴定
色氨酸脱羧酶(TDC,EC 4.1.1.28)催化色氨酸脱羧通过辅因子吡al醛5'-磷酸(PLP)形成色胺,这是喜树碱(CPT)等萜类吲哚生物碱生产的关键阶段。通过转录组分析,从CPT生产植物Ophiorrhiza pumila中鉴定出一个编码TDC的新基因,称为OpTDC2。它包含一个1,536 bp的开放阅读框,编码一个511个氨基酸的蛋白质,分子量为57.01 kDa,等电点为6.39。多序列比对和系统发育树分析显示与Mitragyna speciosa的TDC最接近(85%)。此外,在O. pumila中观察到了OpTDC2的最高表达。根。为了实现OpTDC2在大肠杆菌中的高效表达,我们将TF标签融合到OpTDC2的N端。在45°C,pH 8和0.1 mM的辅因子浓度下观察到最佳的酶活性。Cu 2 +,Zn 2+和Fe 2+的金属离子强烈抑制了催化反应。的升色氨酸被特别催化相比d -色氨酸。此外,OpTDC2的K m和k cat为1.08 mM和0.78 Sec -1, 分别。结果提供了新的功能OpTDC2的信息,该可能在合成生物学中用于增强O. pumila中CPT的生物合成。
更新日期:2020-04-30
中文翻译:

一种来自Ophiorrhiza pumila的新型色氨酸脱羧酶的克隆,表征和酶促鉴定
色氨酸脱羧酶(TDC,EC 4.1.1.28)催化色氨酸脱羧通过辅因子吡al醛5'-磷酸(PLP)形成色胺,这是喜树碱(CPT)等萜类吲哚生物碱生产的关键阶段。通过转录组分析,从CPT生产植物Ophiorrhiza pumila中鉴定出一个编码TDC的新基因,称为OpTDC2。它包含一个1,536 bp的开放阅读框,编码一个511个氨基酸的蛋白质,分子量为57.01 kDa,等电点为6.39。多序列比对和系统发育树分析显示与Mitragyna speciosa的TDC最接近(85%)。此外,在O. pumila中观察到了OpTDC2的最高表达。根。为了实现OpTDC2在大肠杆菌中的高效表达,我们将TF标签融合到OpTDC2的N端。在45°C,pH 8和0.1 mM的辅因子浓度下观察到最佳的酶活性。Cu 2 +,Zn 2+和Fe 2+的金属离子强烈抑制了催化反应。的升色氨酸被特别催化相比d -色氨酸。此外,OpTDC2的K m和k cat为1.08 mM和0.78 Sec -1, 分别。结果提供了新的功能OpTDC2的信息,该可能在合成生物学中用于增强O. pumila中CPT的生物合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号