当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
NIR-Sensitized Activated Photoreaction between Cyanines and Oxime Esters: Free-Radical Photopolymerization.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-04-29 , DOI: 10.1002/anie.202004413 Yulian Pang 1, 2 , Shuheng Fan 2 , Qunying Wang 1 , Dennis Oprych 1 , Alfred Feilen 3 , Knut Reiner 4 , Dietmar Keil 4 , Yuriy L Slominsky 5 , Sergey Popov 6 , Yingquan Zou 2 , Bernd Strehmel 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-04-29 , DOI: 10.1002/anie.202004413 Yulian Pang 1, 2 , Shuheng Fan 2 , Qunying Wang 1 , Dennis Oprych 1 , Alfred Feilen 3 , Knut Reiner 4 , Dietmar Keil 4 , Yuriy L Slominsky 5 , Sergey Popov 6 , Yingquan Zou 2 , Bernd Strehmel 1
Affiliation
|
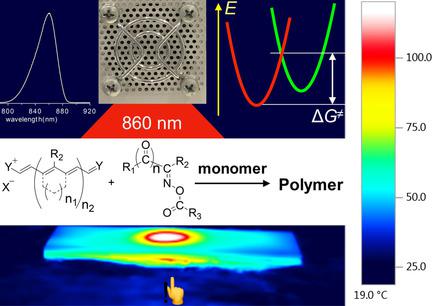
Cyanines comprising either a benzo[e]‐ or benzo[c,d]indolium core facilitate initiation of radical photopolymerization combined with high power NIR‐LED prototypes emitting at 805 nm, 860 nm, or 870 nm, while different oxime esters function as radical coinitiators. Radical photopolymerization followed an initiation mechanism based on the participation of excited states, requiring additional thermal energy to overcome an existing intrinsic activation barrier. Heat released by nonradiative deactivation of the sensitizer favored the system, even under conditions where a thermally activated photoinduced electron transfer controls the reaction protocol. The heat generated internally by the NIR sensitizer promotes generation of the initiating reactive radicals. Sensitizers with a barbiturate group at the meso‐position preferred to bleach directly, while sensitizers carrying a cyclopentene moiety unexpectedly initiated the photosensitized mechanism.
中文翻译:

花青素和肟酯之间的近红外敏化活化光反应:自由基光聚合。
包含苯并[e]-或苯并[c,d]吲哚核的花青酸促进自由基光聚合与在805 nm,860 nm或870 nm处发射的高功率NIR-LED原型结合,而不同的肟酯作为自由基共引发剂。自由基光聚合遵循基于激发态参与的引发机制,需要额外的热能来克服现有的固有活化势垒。即使在热激活的光诱导电子转移控制反应方案的条件下,通过敏化剂的非辐射失活释放的热量也对系统有利。NIR敏化剂内部产生的热量促进引发反应性自由基的产生。在中位具有巴比妥酸盐基团的敏化剂更喜欢直接漂白,
更新日期:2020-07-01
中文翻译:

花青素和肟酯之间的近红外敏化活化光反应:自由基光聚合。
包含苯并[e]-或苯并[c,d]吲哚核的花青酸促进自由基光聚合与在805 nm,860 nm或870 nm处发射的高功率NIR-LED原型结合,而不同的肟酯作为自由基共引发剂。自由基光聚合遵循基于激发态参与的引发机制,需要额外的热能来克服现有的固有活化势垒。即使在热激活的光诱导电子转移控制反应方案的条件下,通过敏化剂的非辐射失活释放的热量也对系统有利。NIR敏化剂内部产生的热量促进引发反应性自由基的产生。在中位具有巴比妥酸盐基团的敏化剂更喜欢直接漂白,



























 京公网安备 11010802027423号
京公网安备 11010802027423号