当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ultrafast amidation of esters using lithium amides under aerobic ambient temperature conditions in sustainable solvents.
Chemical Science ( IF 7.6 ) Pub Date : 2020-04-30 , DOI: 10.1039/d0sc01349h Michael Fairley 1 , Leonie J Bole 2 , Florian F Mulks 1, 2 , Laura Main 1 , Alan R Kennedy 1 , Charles T O'Hara 1 , Joaquín García-Alvarez 3 , Eva Hevia 1, 2
Chemical Science ( IF 7.6 ) Pub Date : 2020-04-30 , DOI: 10.1039/d0sc01349h Michael Fairley 1 , Leonie J Bole 2 , Florian F Mulks 1, 2 , Laura Main 1 , Alan R Kennedy 1 , Charles T O'Hara 1 , Joaquín García-Alvarez 3 , Eva Hevia 1, 2
Affiliation
|
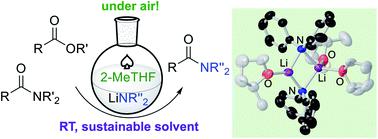
Lithium amides constitute one of the most commonly used classes of reagents in synthetic chemistry. However, despite having many applications, their use is handicapped by the requirement of low temperatures, in order to control their reactivity, as well as the need for dry organic solvents and protective inert atmosphere protocols to prevent their fast decomposition. Advancing the development of air- and moisture-compatible polar organometallic chemistry, the chemoselective and ultrafast amidation of esters mediated by lithium amides is reported. Establishing a novel sustainable access to carboxamides, this has been accomplished via direct C–O bond cleavage of a range of esters using glycerol or 2-MeTHF as a solvent, in air. High yields and good selectivity are observed while operating at ambient temperature, without the need for transition-metal mediation, and the protocol extends to transamidation processes. Pre-coordination of the organic substrate to the reactive lithium amide as a key step in the amidation processes has been assessed, enabling the structural elucidation of the coordination adduct [{Li(NPh2)(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh(NMe2))}2] (8) when toluene is employed as a solvent. No evidence for formation of a complex of this type has been found when using donor THF as a solvent. Structural and spectroscopic insights into the constitution of selected lithium amides in 2-MeTHF are provided that support the involvement of small kinetically activated aggregates that can react rapidly with the organic substrates, favouring the C–O bond cleavage/C–N bond formation processes over competing hydrolysis/degradation of the lithium amides by moisture or air.
CPh(NMe2))}2] (8) when toluene is employed as a solvent. No evidence for formation of a complex of this type has been found when using donor THF as a solvent. Structural and spectroscopic insights into the constitution of selected lithium amides in 2-MeTHF are provided that support the involvement of small kinetically activated aggregates that can react rapidly with the organic substrates, favouring the C–O bond cleavage/C–N bond formation processes over competing hydrolysis/degradation of the lithium amides by moisture or air.
中文翻译:

在有氧环境温度条件下,在可持续溶剂中使用氨基锂对酯进行超快酰胺化。
氨基锂是合成化学中最常用的一类试剂。然而,尽管有许多应用,但它们的使用受到低温要求的限制,以控制它们的反应性,以及需要干燥的有机溶剂和保护性惰性气氛协议来防止它们的快速分解。促进空气和水分相容的极性有机金属化学的发展,报道了由氨基锂介导的酯的化学选择性和超快酰胺化。建立一种新的可持续获取甲酰胺的途径,这是通过以下方式实现的使用甘油或 2-MeTHF 作为溶剂,在空气中直接裂解一系列酯的 C-O 键。在环境温度下操作时观察到高产率和良好的选择性,无需过渡金属调解,并且该协议扩展到转酰胺过程。作为酰胺化过程中的关键步骤,有机底物与活性氨基锂的预配位已被评估,从而能够阐明配位加合物 [{Li(NPh 2 )(O![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh(NMe 2 ))} 2 ]的结构( 8) 当使用甲苯作为溶剂时。当使用供体 THF 作为溶剂时,没有发现形成这种类型复合物的证据。提供了对 2-MeTHF 中选定氨基锂的构成的结构和光谱洞察,支持小的动力学活化聚集体的参与,这些聚集体可以与有机底物快速反应,有利于 C-O 键断裂/C-N 键形成过程水分或空气对氨基锂的竞争性水解/降解。
CPh(NMe 2 ))} 2 ]的结构( 8) 当使用甲苯作为溶剂时。当使用供体 THF 作为溶剂时,没有发现形成这种类型复合物的证据。提供了对 2-MeTHF 中选定氨基锂的构成的结构和光谱洞察,支持小的动力学活化聚集体的参与,这些聚集体可以与有机底物快速反应,有利于 C-O 键断裂/C-N 键形成过程水分或空气对氨基锂的竞争性水解/降解。
更新日期:2020-07-01
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh(NMe2))}2] (8) when toluene is employed as a solvent. No evidence for formation of a complex of this type has been found when using donor THF as a solvent. Structural and spectroscopic insights into the constitution of selected lithium amides in 2-MeTHF are provided that support the involvement of small kinetically activated aggregates that can react rapidly with the organic substrates, favouring the C–O bond cleavage/C–N bond formation processes over competing hydrolysis/degradation of the lithium amides by moisture or air.
CPh(NMe2))}2] (8) when toluene is employed as a solvent. No evidence for formation of a complex of this type has been found when using donor THF as a solvent. Structural and spectroscopic insights into the constitution of selected lithium amides in 2-MeTHF are provided that support the involvement of small kinetically activated aggregates that can react rapidly with the organic substrates, favouring the C–O bond cleavage/C–N bond formation processes over competing hydrolysis/degradation of the lithium amides by moisture or air.
中文翻译:

在有氧环境温度条件下,在可持续溶剂中使用氨基锂对酯进行超快酰胺化。
氨基锂是合成化学中最常用的一类试剂。然而,尽管有许多应用,但它们的使用受到低温要求的限制,以控制它们的反应性,以及需要干燥的有机溶剂和保护性惰性气氛协议来防止它们的快速分解。促进空气和水分相容的极性有机金属化学的发展,报道了由氨基锂介导的酯的化学选择性和超快酰胺化。建立一种新的可持续获取甲酰胺的途径,这是通过以下方式实现的使用甘油或 2-MeTHF 作为溶剂,在空气中直接裂解一系列酯的 C-O 键。在环境温度下操作时观察到高产率和良好的选择性,无需过渡金属调解,并且该协议扩展到转酰胺过程。作为酰胺化过程中的关键步骤,有机底物与活性氨基锂的预配位已被评估,从而能够阐明配位加合物 [{Li(NPh 2 )(O
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh(NMe 2 ))} 2 ]的结构( 8) 当使用甲苯作为溶剂时。当使用供体 THF 作为溶剂时,没有发现形成这种类型复合物的证据。提供了对 2-MeTHF 中选定氨基锂的构成的结构和光谱洞察,支持小的动力学活化聚集体的参与,这些聚集体可以与有机底物快速反应,有利于 C-O 键断裂/C-N 键形成过程水分或空气对氨基锂的竞争性水解/降解。
CPh(NMe 2 ))} 2 ]的结构( 8) 当使用甲苯作为溶剂时。当使用供体 THF 作为溶剂时,没有发现形成这种类型复合物的证据。提供了对 2-MeTHF 中选定氨基锂的构成的结构和光谱洞察,支持小的动力学活化聚集体的参与,这些聚集体可以与有机底物快速反应,有利于 C-O 键断裂/C-N 键形成过程水分或空气对氨基锂的竞争性水解/降解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号