当前位置:
X-MOL 学术
›
BBA Bioenerg.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural dynamics in the C terminal domain homolog of orange carotenoid Protein reveals residues critical for carotenoid uptake.
Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2020-04-30 , DOI: 10.1016/j.bbabio.2020.148214 Dvir Harris 1 , Fernando Muzzopappa 2 , Fabian Glaser 3 , Adjélé Wilson 2 , Diana Kirilovsky 2 , Noam Adir 1
Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2020-04-30 , DOI: 10.1016/j.bbabio.2020.148214 Dvir Harris 1 , Fernando Muzzopappa 2 , Fabian Glaser 3 , Adjélé Wilson 2 , Diana Kirilovsky 2 , Noam Adir 1
Affiliation
|
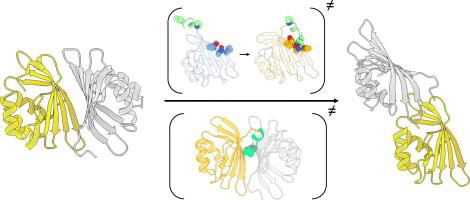
The structural features enabling carotenoid translocation between molecular entities in nature is poorly understood. Here, we present the three-dimensional X-ray structure of an expanded oligomeric state of the C-terminal domain homolog (CTDH) of the orange carotenoid protein, a key water-soluble protein in cyanobacterial photosynthetic photo-protection, at 2.9 Å resolution. This protein binds a canthaxanthin carotenoid ligand and undergoes structural reorganization at the dimeric level, which facilitates cargo uptake and delivery. The structure displays heterogeneity revealing the dynamic nature of its C-terminal tail (CTT). Molecular dynamics (MD) simulations based on the CTDH structures identified specific residues that govern the dimeric transition mechanism. Mutagenesis based on the crystal structure and these MD simulations then confirmed that these specific residues within the CTT are critical for carotenoid uptake, encapsulation and delivery processes. We present a mechanism that can be applied to other systems that require cargo uptake.
中文翻译:

橙色类胡萝卜素蛋白C末端结构域同源物中的结构动力学揭示了对类胡萝卜素摄取至关重要的残基。
人们对自然界中的分子实体之间的类胡萝卜素易位的结构特征了解甚少。在这里,我们介绍了2.9类分辨率的橙色类胡萝卜素蛋白(一种关键的水溶性蛋白,在蓝藻光合光保护中的关键水溶性蛋白)的C端域同源物(CTDH)的扩展低聚状态的三维X射线结构。 。该蛋白与角黄素类胡萝卜素配体结合,并在二聚体水平上进行结构重组,从而促进了货物的吸收和递送。该结构显示出异质性,揭示了其C末端尾巴(CTT)的动态性质。基于CTDH结构的分子动力学(MD)模拟确定了控制二聚体过渡机制的特定残基。然后基于晶体结构和这些MD模拟的诱变证实,CTT中的这些特定残基对于类胡萝卜素的摄取,封装和递送过程至关重要。我们提出了一种机制,该机制可以应用于需要货物吸收的其他系统。
更新日期:2020-04-30
中文翻译:

橙色类胡萝卜素蛋白C末端结构域同源物中的结构动力学揭示了对类胡萝卜素摄取至关重要的残基。
人们对自然界中的分子实体之间的类胡萝卜素易位的结构特征了解甚少。在这里,我们介绍了2.9类分辨率的橙色类胡萝卜素蛋白(一种关键的水溶性蛋白,在蓝藻光合光保护中的关键水溶性蛋白)的C端域同源物(CTDH)的扩展低聚状态的三维X射线结构。 。该蛋白与角黄素类胡萝卜素配体结合,并在二聚体水平上进行结构重组,从而促进了货物的吸收和递送。该结构显示出异质性,揭示了其C末端尾巴(CTT)的动态性质。基于CTDH结构的分子动力学(MD)模拟确定了控制二聚体过渡机制的特定残基。然后基于晶体结构和这些MD模拟的诱变证实,CTT中的这些特定残基对于类胡萝卜素的摄取,封装和递送过程至关重要。我们提出了一种机制,该机制可以应用于需要货物吸收的其他系统。











































 京公网安备 11010802027423号
京公网安备 11010802027423号