当前位置:
X-MOL 学术
›
Drug Metab. Pharmacokinet.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Species Differences in Liver Accumulation and Metabolism of Nucleotide Prodrug Sofosbuvir
Drug Metabolism and Pharmacokinetics ( IF 2.7 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.dmpk.2020.04.333 Ting Wang 1 , Darius Babusis 1 , Yeojin Park 1 , Congrong Niu 1 , Cynthia Kim 1 , Xiaofeng Zhao 1 , Bing Lu 1 , Bin Ma 1 , Robert C Muench 1 , Diana Sperger 1 , Adrian S Ray 1 , Eisuke Murakami 1
Drug Metabolism and Pharmacokinetics ( IF 2.7 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.dmpk.2020.04.333 Ting Wang 1 , Darius Babusis 1 , Yeojin Park 1 , Congrong Niu 1 , Cynthia Kim 1 , Xiaofeng Zhao 1 , Bing Lu 1 , Bin Ma 1 , Robert C Muench 1 , Diana Sperger 1 , Adrian S Ray 1 , Eisuke Murakami 1
Affiliation
|
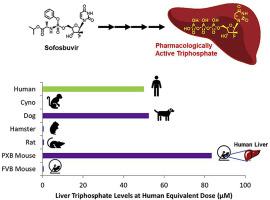
Sofosbuvir (SOF) is a nucleotide prodrug which has been used as a backbone for the clinical treatment of hepatitis C viral infection. Because sofosbuvir undergoes complex first pass metabolism, including metabolic activation to form its pharmacologically active triphosphate (GS-331007-TP) to inhibit the viral RNA polymerase in the liver, it is difficult to project the human dose for clinical evaluation based on preclinical data. Selecting an appropriate animal model for drug exposure in the target tissue is challenging due to differences in absorption, stability, hepatic uptake, and intracellular activation across species. Efficient liver delivery has been established in human liver following administration in a clinical trial of patients receiving sofosbuvir prior to liver transplantation. Using the clinical liver exposure as a benchmark, we assessed and compared the pharmacokinetic profile in mouse, rat, hamster, dog and monkey. Liver accumulation was also assessed in the PXB mouse model in which the liver is mostly populated with human hepatocytes. At human equivalent dose, the hepatic concentrations of GS-331007-TP in dog and PXB mouse were comparable to those observed in the human livers. In these species, high and sustained levels of GS-331007-TP were observed in both primary hepatocytes in vitro and the liver in vivo.
中文翻译:

核苷酸前药Sofosbuvir在肝脏蓄积和代谢中的种属差异
Sofosbuvir (SOF) 是一种核苷酸前药,已被用作临床治疗丙型肝炎病毒感染的骨架。由于 sofosbuvir 经历复杂的首过代谢,包括代谢激活以形成其药理活性三磷酸 (GS-331007-TP) 以抑制肝脏中的病毒 RNA 聚合酶,因此很难根据临床前数据预测用于临床评估的人体剂量。由于不同物种在吸收、稳定性、肝脏摄取和细胞内活化方面存在差异,因此为靶组织中的药物暴露选择合适的动物模型具有挑战性。在肝移植前接受索非布韦的患者的临床试验中,在人类肝脏中建立了有效的肝脏递送。以临床肝脏暴露为基准,我们评估并比较了小鼠、大鼠、仓鼠、狗和猴子的药代动力学特征。还在 PXB 小鼠模型中评估了肝脏积累,其中肝脏主要由人肝细胞填充。在人类等效剂量下,狗和 PXB 小鼠的肝脏 GS-331007-TP 浓度与在人类肝脏中观察到的浓度相当。在这些物种中,在体外的原代肝细胞和体内的肝脏中均观察到高水平且持续的 GS-331007-TP。
更新日期:2020-06-01
中文翻译:

核苷酸前药Sofosbuvir在肝脏蓄积和代谢中的种属差异
Sofosbuvir (SOF) 是一种核苷酸前药,已被用作临床治疗丙型肝炎病毒感染的骨架。由于 sofosbuvir 经历复杂的首过代谢,包括代谢激活以形成其药理活性三磷酸 (GS-331007-TP) 以抑制肝脏中的病毒 RNA 聚合酶,因此很难根据临床前数据预测用于临床评估的人体剂量。由于不同物种在吸收、稳定性、肝脏摄取和细胞内活化方面存在差异,因此为靶组织中的药物暴露选择合适的动物模型具有挑战性。在肝移植前接受索非布韦的患者的临床试验中,在人类肝脏中建立了有效的肝脏递送。以临床肝脏暴露为基准,我们评估并比较了小鼠、大鼠、仓鼠、狗和猴子的药代动力学特征。还在 PXB 小鼠模型中评估了肝脏积累,其中肝脏主要由人肝细胞填充。在人类等效剂量下,狗和 PXB 小鼠的肝脏 GS-331007-TP 浓度与在人类肝脏中观察到的浓度相当。在这些物种中,在体外的原代肝细胞和体内的肝脏中均观察到高水平且持续的 GS-331007-TP。











































 京公网安备 11010802027423号
京公网安备 11010802027423号