当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reiterative Chiral Resolution/Racemization/Recycle (RRR Synthesis) for an Effective and Scalable Process for the Enantioselective Synthesis of a Dual IDO1/TDO2 Inhibitor Imidazoisoindole Derivative
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-04-30 , DOI: 10.1021/acs.oprd.0c00004 Cristina Crescenzi 1 , Thomas Fuchss 2 , Dimitri Ippoliti 1 , Annunziata Langella 1 , Antonia Di Mola 3 , Antonio Massa 3 , Diego Rozzi 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-04-30 , DOI: 10.1021/acs.oprd.0c00004 Cristina Crescenzi 1 , Thomas Fuchss 2 , Dimitri Ippoliti 1 , Annunziata Langella 1 , Antonia Di Mola 3 , Antonio Massa 3 , Diego Rozzi 1
Affiliation
|
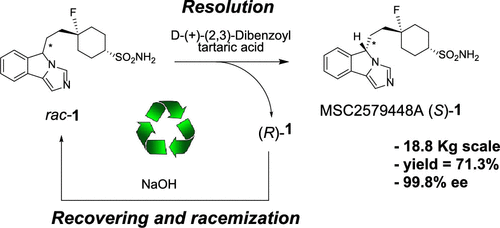
Herein, we report an effective and scalable highly enantioselective synthesis of an 5H-imidazo[5,1-a]isoindole derivative via an RRR-synthesis approach (resolution–racemization–recycle). Chiral resolution performed with the aid of 2,3-dibenzoyl-d-tartaric acid allowed the obtaining of the desired enantiomer in high enantiopurity (>99% ee) and good yield. The undesired enantiomer was subjected to racemization under basic conditions and again to resolution, improving the efficiency of the entire process. The asymmetric formation of the new stereocenter in an early stage of the synthesis scheme was also investigated by means of organocatalytic systems.
中文翻译:

重复手性拆分/对映/循环(RRR合成),可实现对IDO1 / TDO2双重抑制剂咪唑异吲哚衍生物的对映选择性合成的有效且可扩展的过程
在本文中,我们报告了一种有效且可扩展的5R-咪唑并[5,1- a ]异吲哚衍生物的高对映选择性合成方法,其合成方法为:RRR(拆分-消旋-循环)。手性拆分用的2,3-二苯甲酰基帮助下执行d -酒石酸允许高对映体纯度(> 99%ee)和良好产率的所需对映体的获得。不需要的对映异构体在碱性条件下外消旋,并再次拆分,从而提高了整个过程的效率。还通过有机催化体系研究了合成方案早期新立体中心的不对称形成。
更新日期:2020-06-19
中文翻译:

重复手性拆分/对映/循环(RRR合成),可实现对IDO1 / TDO2双重抑制剂咪唑异吲哚衍生物的对映选择性合成的有效且可扩展的过程
在本文中,我们报告了一种有效且可扩展的5R-咪唑并[5,1- a ]异吲哚衍生物的高对映选择性合成方法,其合成方法为:RRR(拆分-消旋-循环)。手性拆分用的2,3-二苯甲酰基帮助下执行d -酒石酸允许高对映体纯度(> 99%ee)和良好产率的所需对映体的获得。不需要的对映异构体在碱性条件下外消旋,并再次拆分,从而提高了整个过程的效率。还通过有机催化体系研究了合成方案早期新立体中心的不对称形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号